
最新刊期
卷 49 , 期 8 , 2024
-
 摘要:To address the issue of low dispersion of Cu species in Cu-based catalysts used in the synthesis of low-carbon alcohols from syngas, the structures of [Cu(EDTA)]2- intercalated ZnAl hydrotalcite-like catalysts were successfully adjusted by four intercalation methods, including coprecipitation, ion exchange, calcination recovery, and reprecipitation methods. The catalyst structures were characterized by XRD, N2 physical adsorption/desorption, TEM, H2-TPR, XPS and so on, and the catalytic performances of catalysts in CO hydrogenation to low-carbon alcohols were also investigated. The results show that the Cu dispersities, Cu specific surface areas and the relative contents of oxygen vacancy are regulated by the intercalation methods, thereby affecting their catalytic performances. The catalyst prepared by calcination recovery method exhibites the highest Cu dispersity, the largest Cu specific surface area and the highest oxygen vacancy concentration. Moreover, the interaction between Cu species and ZnO on the layered is strong. This catalyst exhibites the best catalytic performance under reaction conditions of temperature 280 ℃, pressure 4.0 MPa, gas hourly space velocity 2000 h-1 and V(H2):V(CO) = 1:1, and the total alcohol space-time yield achieves 17468.7 mg/(g·h).关键词:[Cu(EDTA)]2-;hydrotalcite-like compounds;dispersity;syngas;low-carbon alcohols
摘要:To address the issue of low dispersion of Cu species in Cu-based catalysts used in the synthesis of low-carbon alcohols from syngas, the structures of [Cu(EDTA)]2- intercalated ZnAl hydrotalcite-like catalysts were successfully adjusted by four intercalation methods, including coprecipitation, ion exchange, calcination recovery, and reprecipitation methods. The catalyst structures were characterized by XRD, N2 physical adsorption/desorption, TEM, H2-TPR, XPS and so on, and the catalytic performances of catalysts in CO hydrogenation to low-carbon alcohols were also investigated. The results show that the Cu dispersities, Cu specific surface areas and the relative contents of oxygen vacancy are regulated by the intercalation methods, thereby affecting their catalytic performances. The catalyst prepared by calcination recovery method exhibites the highest Cu dispersity, the largest Cu specific surface area and the highest oxygen vacancy concentration. Moreover, the interaction between Cu species and ZnO on the layered is strong. This catalyst exhibites the best catalytic performance under reaction conditions of temperature 280 ℃, pressure 4.0 MPa, gas hourly space velocity 2000 h-1 and V(H2):V(CO) = 1:1, and the total alcohol space-time yield achieves 17468.7 mg/(g·h).关键词:[Cu(EDTA)]2-;hydrotalcite-like compounds;dispersity;syngas;low-carbon alcohols- 29
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:Fe-based and Co-based catalysts are the most ideal Fischer-Tropsch synthesis catalysts. With the rapid development of computer simulation technology in the field of catalysis, it is possible to deeply understand the microscopic reaction mechanism on the surface of Fe-based and Co-based catalysts. Theoretical calculation method was used to study the effects of surface structures of Fe-based (reaction conditions: temperature 320 ℃, pressure 1 MPa and n(H2):n(CO) = 1:1) and Co-based (reaction conditions: temperature 240 ℃, pressure 1 MPa and n(H2):n(CO) = 2:1) Fischer-Tropsch synthesis catalysts with different H coverages on the activation and dissociation of CO. The thermodynamically preferred H-covered surface models of Fe-based and Co-based Fischer-Tropsch synthesis catalysts were determined through the linear relationships between the hydrogen chemical potential and the Gibbs free energy of hydrogen adsorption, which is the χ-Fe5C2(510) surface with H coverage of 11/42 and HCP Co(0001) surface with H coverage of 24/36, respectively. The CO dissociation mechanism and corresponding reaction energy barriers on the clean surface and the H-covered surface of catalysts were studied, and it is found that H coverage can change the reaction pathways of CO dissociation and increase the dissociation energy barrier. Compared with the clean surfaces of χ-Fe5C2(510) and HCP Co(0001), the dissociation energy barriers of CO on H-covered χ-Fe5C2(510) and HCP Co(0001) surfaces increase by 5.4% and 20.3%, respectively. The origin reasons for the effects of H coverage on CO activation and dissociation were explained by calculating the density of states and crystal orbitals Hamiltonian population.关键词:Fischer-Tropsch synthesis catalysts;CO activation;H coverage;DFT calculation
摘要:Fe-based and Co-based catalysts are the most ideal Fischer-Tropsch synthesis catalysts. With the rapid development of computer simulation technology in the field of catalysis, it is possible to deeply understand the microscopic reaction mechanism on the surface of Fe-based and Co-based catalysts. Theoretical calculation method was used to study the effects of surface structures of Fe-based (reaction conditions: temperature 320 ℃, pressure 1 MPa and n(H2):n(CO) = 1:1) and Co-based (reaction conditions: temperature 240 ℃, pressure 1 MPa and n(H2):n(CO) = 2:1) Fischer-Tropsch synthesis catalysts with different H coverages on the activation and dissociation of CO. The thermodynamically preferred H-covered surface models of Fe-based and Co-based Fischer-Tropsch synthesis catalysts were determined through the linear relationships between the hydrogen chemical potential and the Gibbs free energy of hydrogen adsorption, which is the χ-Fe5C2(510) surface with H coverage of 11/42 and HCP Co(0001) surface with H coverage of 24/36, respectively. The CO dissociation mechanism and corresponding reaction energy barriers on the clean surface and the H-covered surface of catalysts were studied, and it is found that H coverage can change the reaction pathways of CO dissociation and increase the dissociation energy barrier. Compared with the clean surfaces of χ-Fe5C2(510) and HCP Co(0001), the dissociation energy barriers of CO on H-covered χ-Fe5C2(510) and HCP Co(0001) surfaces increase by 5.4% and 20.3%, respectively. The origin reasons for the effects of H coverage on CO activation and dissociation were explained by calculating the density of states and crystal orbitals Hamiltonian population.关键词:Fischer-Tropsch synthesis catalysts;CO activation;H coverage;DFT calculation- 24
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:The preparation of high value-added chemicals via CO hydrogenation with Fischer-Tropsch synthesis is one of the important ways to achieve clean and efficient utilization of coal. The development of efficient catalysts is the key to achieve targeted regulation of product distributions, improve the selectivity of target products and inhibit the formation of C1 by-products. The surface properties of catalysts have a significant impact on the adsorption and activation of CO, hydrogenation activation and product distributions. Anhydrous glucose was used as the carbon source and ethylene glycol was used as the solvent to prepare the carbon support by solvothermal method. After H2O2 and NH3•H2O surface modification and nitrogen doping, 5Fe/CS-H2O2, 5Fe/CS-NH3•H2O and 5Fe/CS-N catalysts were prepared by impregnation method, respectively. The effect of surface modification on the product distributions of Fischer-Tropsch synthesis was investigated. The samples were characterized by XRD, SEM, TEM, N2 adsorption/desorption, TG-DTG, FT-IR, Zeta potential, XPS and Raman. The results show that different surface modification methods have a significant impact on the surface physicochemical properties and catalytic performances of catalysts. The modification of H2O2 increases the amount of —OH on catalyst surface, enhances the hydrophilicity of catalyst surface, promotes the dispersion of Fe on the support, and improves the thermal stability of catalysts. The activity test of CO hydrogenation reaction was conducted under the conditions of 300 ℃, 1.5 MPa, space velocity 1000 h-1 and n(H2):n(CO) = 2. The results show that the modified catalysts significantly inhibit the generation of CH4 and improve the selectivity of light olefins. The olefin selectivities of 5Fe/CS-NH3•H2O, 5Fe/CS-N and 5Fe/CS-H2O2 increase from 24.39% to 34.94%, 37.63% and 43.57%, respectively. The surface properties of the catalyst are adjusted by surface modification and nitrogen-doping treatment, and the product distribution is optimized.关键词:CO hydrogenation;Fe/CS catalysts;surface modification;light olefins
摘要:The preparation of high value-added chemicals via CO hydrogenation with Fischer-Tropsch synthesis is one of the important ways to achieve clean and efficient utilization of coal. The development of efficient catalysts is the key to achieve targeted regulation of product distributions, improve the selectivity of target products and inhibit the formation of C1 by-products. The surface properties of catalysts have a significant impact on the adsorption and activation of CO, hydrogenation activation and product distributions. Anhydrous glucose was used as the carbon source and ethylene glycol was used as the solvent to prepare the carbon support by solvothermal method. After H2O2 and NH3•H2O surface modification and nitrogen doping, 5Fe/CS-H2O2, 5Fe/CS-NH3•H2O and 5Fe/CS-N catalysts were prepared by impregnation method, respectively. The effect of surface modification on the product distributions of Fischer-Tropsch synthesis was investigated. The samples were characterized by XRD, SEM, TEM, N2 adsorption/desorption, TG-DTG, FT-IR, Zeta potential, XPS and Raman. The results show that different surface modification methods have a significant impact on the surface physicochemical properties and catalytic performances of catalysts. The modification of H2O2 increases the amount of —OH on catalyst surface, enhances the hydrophilicity of catalyst surface, promotes the dispersion of Fe on the support, and improves the thermal stability of catalysts. The activity test of CO hydrogenation reaction was conducted under the conditions of 300 ℃, 1.5 MPa, space velocity 1000 h-1 and n(H2):n(CO) = 2. The results show that the modified catalysts significantly inhibit the generation of CH4 and improve the selectivity of light olefins. The olefin selectivities of 5Fe/CS-NH3•H2O, 5Fe/CS-N and 5Fe/CS-H2O2 increase from 24.39% to 34.94%, 37.63% and 43.57%, respectively. The surface properties of the catalyst are adjusted by surface modification and nitrogen-doping treatment, and the product distribution is optimized.关键词:CO hydrogenation;Fe/CS catalysts;surface modification;light olefins- 18
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:With the decrease of crude oil reserves and the aggravation of environmental problems, it is urgent to find new technologies for producing fuels and chemicals. Cu-based catalyst is one of the important catalysts for the direct synthesis of ethanol and higher alcohols (C2+ alcohols) from syngas, but it has the problem of low selectivity for target products. A series of CuZnAl catalysts (Cat-Alx) with different Al component contents (n(Al)/n(Zn)) were prepared by the complete liquid-phase method. The phase compositions, texture properties and reduction properties of the catalysts were characterized by X-ray diffraction, N2 adsorption/desorption and H2-programmed temperature reduction, etc. The catalytic performances of Cat-Alx for CO hydrogenation to ethanol and C2+ alcohols were studied in a simulated slurry bed reactor. The results show that Cat-Al0.8 has the most abundant Cu+ species and more oxygen vacancies on its surface, showing relatively optimal catalytic performance. Reaction under the conditions of 280 ℃, 4 MPa and V(H2)/V(CO) = 2/1 for 24 h, the CO conversion rate of Cat-Al0.8 is 18.45%, and the total alcohols selectivity is 30.34%, and the proportion of ethanol in the total alcohols is 30.19%, and the proportion of C2+ alcohols in the total alcohols is 48.08%.关键词:Al component contents;CuZnAl catalysts;complete liquid-phase method;ethanol;higher alcohols
摘要:With the decrease of crude oil reserves and the aggravation of environmental problems, it is urgent to find new technologies for producing fuels and chemicals. Cu-based catalyst is one of the important catalysts for the direct synthesis of ethanol and higher alcohols (C2+ alcohols) from syngas, but it has the problem of low selectivity for target products. A series of CuZnAl catalysts (Cat-Alx) with different Al component contents (n(Al)/n(Zn)) were prepared by the complete liquid-phase method. The phase compositions, texture properties and reduction properties of the catalysts were characterized by X-ray diffraction, N2 adsorption/desorption and H2-programmed temperature reduction, etc. The catalytic performances of Cat-Alx for CO hydrogenation to ethanol and C2+ alcohols were studied in a simulated slurry bed reactor. The results show that Cat-Al0.8 has the most abundant Cu+ species and more oxygen vacancies on its surface, showing relatively optimal catalytic performance. Reaction under the conditions of 280 ℃, 4 MPa and V(H2)/V(CO) = 2/1 for 24 h, the CO conversion rate of Cat-Al0.8 is 18.45%, and the total alcohols selectivity is 30.34%, and the proportion of ethanol in the total alcohols is 30.19%, and the proportion of C2+ alcohols in the total alcohols is 48.08%.关键词:Al component contents;CuZnAl catalysts;complete liquid-phase method;ethanol;higher alcohols- 27
- |
- 0
- |
- 0
发布时间:2024-09-02 -
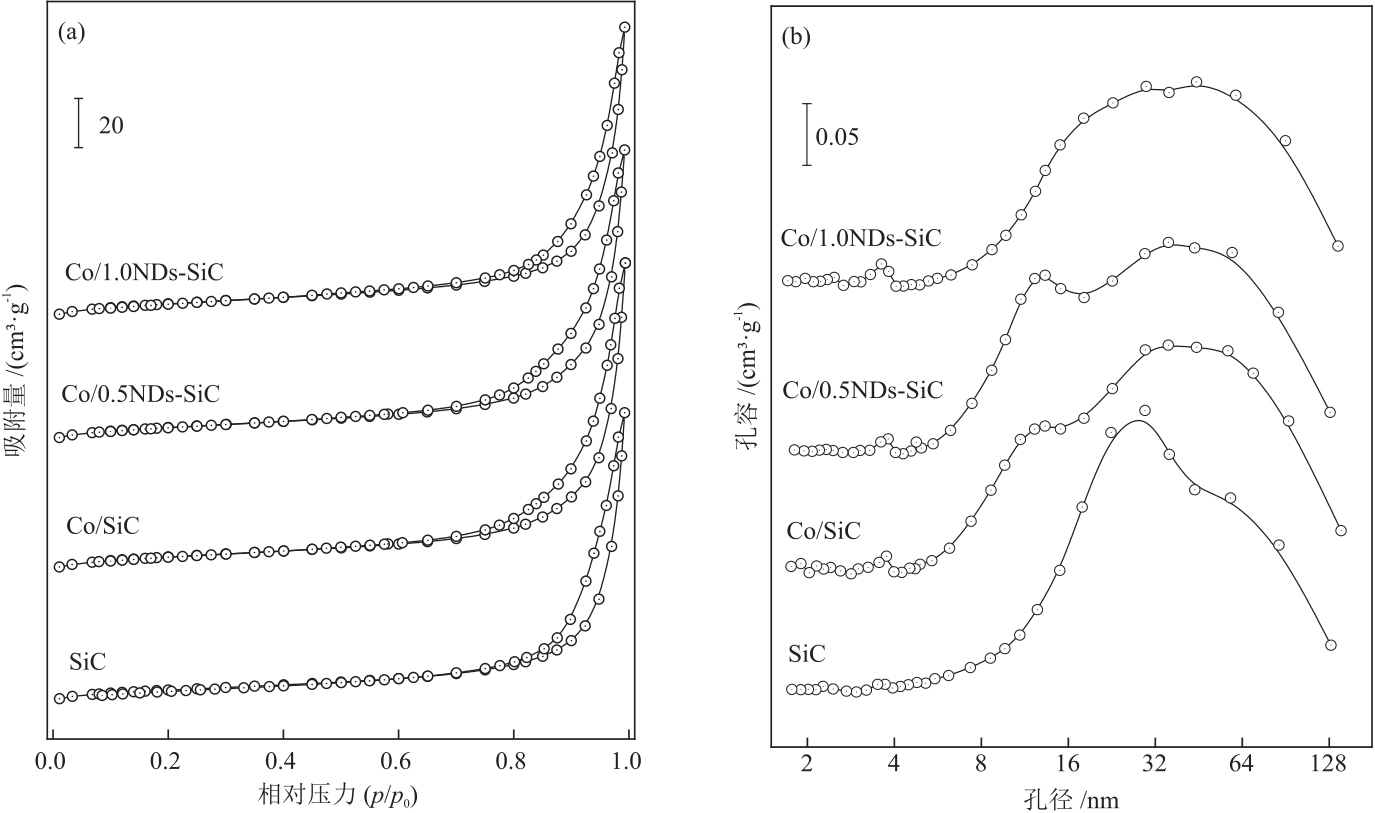 摘要:Cobalt-based catalysts exhibit excellent reactivity and carbon chain growth capability in the Fischer-Tropsch synthesis reaction, and their catalytic performance is closely related to the reduction degree and size of metal Co particles. Regulating the reduction degree and dispersion degree of metal Co by adding promoters is an important way to enhance their catalytic performance in Fischer-Tropsch synthesis reaction. By using SiC as a support with chemical inertness and good thermal conductivity, and adding a small amount of nanodiamonds (NDs) with rich surface functional groups as structural regulators, the dispersion degree and reduction degree of Co species in the catalyst were effectively improved, and its catalytic performance in Fischer-Tropsch synthesis reaction was enhanced. The results of N2 physical adsorption/desorption show that the addition of NDs has little effect on the parameters such as specific surface areas and pore sizes of the catalysts. The results of CO chemisorption show that the addition of an appropriate amount of NDs promotes the dispersion of active metal Co in the catalysts, increases the surface area of metal Co and provides more active sites for the Fischer-Tropsch synthesis reaction. The results of transmission electron microscopy (TEM) show that after the addition of NDs to Co/SiC (m(NDs):m(SiC) = 1.0%), the particle sizes of metal Co in the catalysts are reduced, and NDs are uniformly dispersed on the catalyst surface. The results of H2-temperature programmed reduction (H2-TPR) and CO-H2-temperature programmed surface reaction (CO-H2-TPSR) show that the addition of NDs reduces the reduction temperature of Co3O4 to metal Co and promotes the reduction of Co3O4 and the activation of CO. Compared with the Co/SiC catalyst, the NDs-modified Co/1.0NDs-SiC (w(Co) = 10%, m(NDs):m(SiC) = 1.0%) catalyst exhibits an increase of CO conversion rate from 15.6% to 29.0%, and the selectivity of C5+ hydrocarbon products still maintains at above 75%, and shows stable operation and no significant deactivation for 70 h. The above results can provide a new research idea for the development of efficient catalysts modified with nanocarbon materials in Fischer-Tropsch synthesis reaction.关键词:Co-based catalyst;Fischer-Tropsch synthesis;nanodiamonds;carbon regulator
摘要:Cobalt-based catalysts exhibit excellent reactivity and carbon chain growth capability in the Fischer-Tropsch synthesis reaction, and their catalytic performance is closely related to the reduction degree and size of metal Co particles. Regulating the reduction degree and dispersion degree of metal Co by adding promoters is an important way to enhance their catalytic performance in Fischer-Tropsch synthesis reaction. By using SiC as a support with chemical inertness and good thermal conductivity, and adding a small amount of nanodiamonds (NDs) with rich surface functional groups as structural regulators, the dispersion degree and reduction degree of Co species in the catalyst were effectively improved, and its catalytic performance in Fischer-Tropsch synthesis reaction was enhanced. The results of N2 physical adsorption/desorption show that the addition of NDs has little effect on the parameters such as specific surface areas and pore sizes of the catalysts. The results of CO chemisorption show that the addition of an appropriate amount of NDs promotes the dispersion of active metal Co in the catalysts, increases the surface area of metal Co and provides more active sites for the Fischer-Tropsch synthesis reaction. The results of transmission electron microscopy (TEM) show that after the addition of NDs to Co/SiC (m(NDs):m(SiC) = 1.0%), the particle sizes of metal Co in the catalysts are reduced, and NDs are uniformly dispersed on the catalyst surface. The results of H2-temperature programmed reduction (H2-TPR) and CO-H2-temperature programmed surface reaction (CO-H2-TPSR) show that the addition of NDs reduces the reduction temperature of Co3O4 to metal Co and promotes the reduction of Co3O4 and the activation of CO. Compared with the Co/SiC catalyst, the NDs-modified Co/1.0NDs-SiC (w(Co) = 10%, m(NDs):m(SiC) = 1.0%) catalyst exhibits an increase of CO conversion rate from 15.6% to 29.0%, and the selectivity of C5+ hydrocarbon products still maintains at above 75%, and shows stable operation and no significant deactivation for 70 h. The above results can provide a new research idea for the development of efficient catalysts modified with nanocarbon materials in Fischer-Tropsch synthesis reaction.关键词:Co-based catalyst;Fischer-Tropsch synthesis;nanodiamonds;carbon regulator- 34
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:The photocatalytic partial oxidation of CH4 to CH3OH, HCHO and other products is a potential low-energy CH4 conversion path, which has the problems of high activation difficulty of CH4 and low product selectivity. The design and preparation of high-performance catalysts are crucial to solve these problems. Based on the hydrothermal method, ZnO-x catalysts with different morphologies including granules, nano-flower, nano-flake and agglomerated nanoparticles were prepared by varying Zn precursors. It is found that the morphologies of ZnO-x has a significant effect on photocatalytic partial oxidation of CH4 to HCHO (short for “CH4 photocatalytic oxidation performance”). The nAgCl/ZnO-Cl photocatalysts (n is the mole fraction of AgNO3) were further prepared by using ZnO-Cl as the substrate with the best CH4 photocatalytic oxidation performance. The samples were characterized by X-ray diffraction, N2 adsorption/desorption and scanning electron microscopy, etc. The results show that 2.0%AgCl/ZnO-Cl shows the best CH4 photocatalytic oxidation performance. Under the action of 5 mg 2.0%AgCl/ZnO-Cl and reaction conditions of temperature of 25 ℃, O2 pressure of 0.1 MPa, CH4 pressure of 2.9 MPa, H2O volume of 75 mL, light intensity of 450 mW/cm2 and illumination time of 2 h. The total yield of oxygenated liquid products (CH3OH + CH3OOH + HCHO) is 10409 μmol/(g·h), and the total selectivity of oxygenated liquid products is 91.4%, and the main product HCHO is 6271 μmol/(g·h) with HCHO selectivity of 60.2%. Based on radical trapping experiments, it is found that the formation of •O2- and the holes are the key to the activation of CH4 to •CH3, and more importantly, interaction of •O2- and •CH3 becomes the major reaction path. In such a way, the initial product (CH3OOH) can be further oxidized to HCHO, and thus leading to the promotion of HCHO selectivity of nAgCl/ZnO-Cl.关键词:CH4;photocatalytic partial oxidation;ZnO;HCHO
摘要:The photocatalytic partial oxidation of CH4 to CH3OH, HCHO and other products is a potential low-energy CH4 conversion path, which has the problems of high activation difficulty of CH4 and low product selectivity. The design and preparation of high-performance catalysts are crucial to solve these problems. Based on the hydrothermal method, ZnO-x catalysts with different morphologies including granules, nano-flower, nano-flake and agglomerated nanoparticles were prepared by varying Zn precursors. It is found that the morphologies of ZnO-x has a significant effect on photocatalytic partial oxidation of CH4 to HCHO (short for “CH4 photocatalytic oxidation performance”). The nAgCl/ZnO-Cl photocatalysts (n is the mole fraction of AgNO3) were further prepared by using ZnO-Cl as the substrate with the best CH4 photocatalytic oxidation performance. The samples were characterized by X-ray diffraction, N2 adsorption/desorption and scanning electron microscopy, etc. The results show that 2.0%AgCl/ZnO-Cl shows the best CH4 photocatalytic oxidation performance. Under the action of 5 mg 2.0%AgCl/ZnO-Cl and reaction conditions of temperature of 25 ℃, O2 pressure of 0.1 MPa, CH4 pressure of 2.9 MPa, H2O volume of 75 mL, light intensity of 450 mW/cm2 and illumination time of 2 h. The total yield of oxygenated liquid products (CH3OH + CH3OOH + HCHO) is 10409 μmol/(g·h), and the total selectivity of oxygenated liquid products is 91.4%, and the main product HCHO is 6271 μmol/(g·h) with HCHO selectivity of 60.2%. Based on radical trapping experiments, it is found that the formation of •O2- and the holes are the key to the activation of CH4 to •CH3, and more importantly, interaction of •O2- and •CH3 becomes the major reaction path. In such a way, the initial product (CH3OOH) can be further oxidized to HCHO, and thus leading to the promotion of HCHO selectivity of nAgCl/ZnO-Cl.关键词:CH4;photocatalytic partial oxidation;ZnO;HCHO- 29
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:The methane dry reforming (DRM) reaction is of great significance for the co-conversion and utilization of greenhouse gases CO2 and CH4. However, this reaction faces the crucial problem of catalyst deactivation caused by carbon deposition. The influence of support morphologies on the reaction performance in DRM over Ni-based catalysts was investigated by preparing flower-shaped, granular and sheet-like MgO supported catalysts. The influence mechanisms were clarified by various characterizations such as XRD, SEM, H2-TPR, CO2-TPD and TG. The results show that under the temperature of 800 °C and space velocity of 54000 mL/(g·h), the average CO2 and CH4 conversion rates of Ni-based catalysts supported on flower-shaped MgO reach 90.2% and 82.3%, respectively, with the average n(H2)/n(CO) of 0.93 after 50 h reaction. Compared with Ni-based catalysts supported on granular and sheet-like MgO, the Ni-based catalyst supported on flower-shaped MgO exhibits higher activity, stability and anti-carbon deposition ability. This is because the flower-shaped MgO has a higher specific surface area favorable for the dispersion of active metals, and there are more alkaline sites on the surface of flower-shaped MgO, which contribute to the adsorption and activation of CO2, enhance the anti-carbon deposition ability of catalysts and improve the activity and stability of DRM reaction.关键词:methane dry reforming;support morphology;Ni-based catalyst;carbon deposition;catalytic performance
摘要:The methane dry reforming (DRM) reaction is of great significance for the co-conversion and utilization of greenhouse gases CO2 and CH4. However, this reaction faces the crucial problem of catalyst deactivation caused by carbon deposition. The influence of support morphologies on the reaction performance in DRM over Ni-based catalysts was investigated by preparing flower-shaped, granular and sheet-like MgO supported catalysts. The influence mechanisms were clarified by various characterizations such as XRD, SEM, H2-TPR, CO2-TPD and TG. The results show that under the temperature of 800 °C and space velocity of 54000 mL/(g·h), the average CO2 and CH4 conversion rates of Ni-based catalysts supported on flower-shaped MgO reach 90.2% and 82.3%, respectively, with the average n(H2)/n(CO) of 0.93 after 50 h reaction. Compared with Ni-based catalysts supported on granular and sheet-like MgO, the Ni-based catalyst supported on flower-shaped MgO exhibits higher activity, stability and anti-carbon deposition ability. This is because the flower-shaped MgO has a higher specific surface area favorable for the dispersion of active metals, and there are more alkaline sites on the surface of flower-shaped MgO, which contribute to the adsorption and activation of CO2, enhance the anti-carbon deposition ability of catalysts and improve the activity and stability of DRM reaction.关键词:methane dry reforming;support morphology;Ni-based catalyst;carbon deposition;catalytic performance- 30
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:The embedded catalysts can effectively prevent the sintering behavior, and realize the high activity in partial oxidation of methane (POM) reaction. The embedded Ni@SiO2 catalysts were prepared by Stöber method, and the Al, Ti or Zr were used to modify them. Various characterizations, such as X-ray diffraction (XRD), transmission electron microscope (TEM) and N2 adsorption/desorption, were employed to the characterizations of crystalline phase structures, morphologies and texture parameters of catalysts. The effects of modification of catalysts on the catalytic POM performance were further investigated under the feed gases compositions of V(CH4):V(O2):V(N2) of 2:1:3, gas flow rate of 60 mL/min, pressure of 0.1 MPa, space velocity of 7.2 L/(g·h) and reaction time of 22 h. The results show that compared with Ni@SiO2 catalyst, the Ni@Al-SiO2 catalyst can promote the activation of methane and its catalytic performance is improved. However, the Ni@Ti-SiO2 and Ni@Zr-SiO2 catalysts show the decreased catalytic performances because of the obstruction of active sites. At 700 ℃, the CH4 conversation rates of Ni@Al-SiO2 and Ni@SiO2 are 86% and 80%, the CO selectivities are both about 90%, and the H2 selectivities are 93% and 88%, respectively. However, compared with Ni@SiO2 catalyst, the CH4 conversion rates, CO selectivities and H2 selectivities of Ni@Ti-SiO2 and Ni@Zr-SiO2 are significantly reduced. It reveals that the catalyst deactivation can be mainly ascribed to the loss of active sites and carbon deposit. But the active sites are not completely covered by deposited carbon, and the catalyst still maintain robust catalytic POM performance.关键词:Ni@SiO2 catalysts;embedded structure;catalyst modification;partial oxidation of methane;carbon deposit
摘要:The embedded catalysts can effectively prevent the sintering behavior, and realize the high activity in partial oxidation of methane (POM) reaction. The embedded Ni@SiO2 catalysts were prepared by Stöber method, and the Al, Ti or Zr were used to modify them. Various characterizations, such as X-ray diffraction (XRD), transmission electron microscope (TEM) and N2 adsorption/desorption, were employed to the characterizations of crystalline phase structures, morphologies and texture parameters of catalysts. The effects of modification of catalysts on the catalytic POM performance were further investigated under the feed gases compositions of V(CH4):V(O2):V(N2) of 2:1:3, gas flow rate of 60 mL/min, pressure of 0.1 MPa, space velocity of 7.2 L/(g·h) and reaction time of 22 h. The results show that compared with Ni@SiO2 catalyst, the Ni@Al-SiO2 catalyst can promote the activation of methane and its catalytic performance is improved. However, the Ni@Ti-SiO2 and Ni@Zr-SiO2 catalysts show the decreased catalytic performances because of the obstruction of active sites. At 700 ℃, the CH4 conversation rates of Ni@Al-SiO2 and Ni@SiO2 are 86% and 80%, the CO selectivities are both about 90%, and the H2 selectivities are 93% and 88%, respectively. However, compared with Ni@SiO2 catalyst, the CH4 conversion rates, CO selectivities and H2 selectivities of Ni@Ti-SiO2 and Ni@Zr-SiO2 are significantly reduced. It reveals that the catalyst deactivation can be mainly ascribed to the loss of active sites and carbon deposit. But the active sites are not completely covered by deposited carbon, and the catalyst still maintain robust catalytic POM performance.关键词:Ni@SiO2 catalysts;embedded structure;catalyst modification;partial oxidation of methane;carbon deposit- 28
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:CO2 hydrogenation to methane (also known as “CO2 methanation”) is an important way to achieve the goal of carbon neutralization. CeO2 is considered as one of the most important catalyst supports because of its abundant surface oxygen vacancies and excellent oxygen storage capacity. Transition metal Ni is also widely studied because of its excellent catalytic performance and low price. The Ni/CeO2-based catalysts formed by the combination of CeO2 and metal Ni show good application prospects in CO2 methanation. The reaction mechanisms of Ni/CeO2-based catalysts for CO2 methanation were summarized and the preparation methods of Ni/CeO2-based catalysts were introduced, and the key parameters affecting catalytic performance of CO2 methanation such as the characteristics of active centers, properties of supports, types of additives and metal-support interactions were summarized, and the catalytic performances of Ni/CeO2-based catalysts were also summarized. It is found that by modifying Ni/CeO2-based catalysts, the catalytic performances and product selectivities of Ni/CeO2-based catalysts for CO2 methanation can be regulated, which can provide new ideas for improving their catalytic performances of CO2 methanation.关键词:Ni/CeO2-based catalysts;CO2 methanation;metal-support interactions;preparation method
摘要:CO2 hydrogenation to methane (also known as “CO2 methanation”) is an important way to achieve the goal of carbon neutralization. CeO2 is considered as one of the most important catalyst supports because of its abundant surface oxygen vacancies and excellent oxygen storage capacity. Transition metal Ni is also widely studied because of its excellent catalytic performance and low price. The Ni/CeO2-based catalysts formed by the combination of CeO2 and metal Ni show good application prospects in CO2 methanation. The reaction mechanisms of Ni/CeO2-based catalysts for CO2 methanation were summarized and the preparation methods of Ni/CeO2-based catalysts were introduced, and the key parameters affecting catalytic performance of CO2 methanation such as the characteristics of active centers, properties of supports, types of additives and metal-support interactions were summarized, and the catalytic performances of Ni/CeO2-based catalysts were also summarized. It is found that by modifying Ni/CeO2-based catalysts, the catalytic performances and product selectivities of Ni/CeO2-based catalysts for CO2 methanation can be regulated, which can provide new ideas for improving their catalytic performances of CO2 methanation.关键词:Ni/CeO2-based catalysts;CO2 methanation;metal-support interactions;preparation method- 58
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:Catalytic hydrogenation of carbon dioxide (CO2) to higher alcohols is one of the important ways to realize the high value utilization of CO2 and mitigate the greenhouse effect. The research progress on catalytic hydrogenation of CO2 to higher alcohols in recent years was reviewed. Firstly, the favorable reaction conditions and catalyst requirements for catalytic hydrogenation of CO2 to higher alcohols were analyzed from the thermodynamic point of view. Then the researches of different types of catalysts (precious metal based, copper based, cobalt and molybdenum based catalysts) applicable to the field were introduced. Finally, based on the key intermediates and main reaction pathways, the synthesis mechanisms of catalytic hydrogenation of CO2 to higher alcohols were summarized. Through summary and analysis, the main challenges in the research of catalytic hydrogenation of CO2 to higher alcohols were pointed out, and the future research direction in the field was prospected.关键词:carbon dioxide;catalytic hydrogenation;higher alcohols;catalysts;synthesis mechanisms
摘要:Catalytic hydrogenation of carbon dioxide (CO2) to higher alcohols is one of the important ways to realize the high value utilization of CO2 and mitigate the greenhouse effect. The research progress on catalytic hydrogenation of CO2 to higher alcohols in recent years was reviewed. Firstly, the favorable reaction conditions and catalyst requirements for catalytic hydrogenation of CO2 to higher alcohols were analyzed from the thermodynamic point of view. Then the researches of different types of catalysts (precious metal based, copper based, cobalt and molybdenum based catalysts) applicable to the field were introduced. Finally, based on the key intermediates and main reaction pathways, the synthesis mechanisms of catalytic hydrogenation of CO2 to higher alcohols were summarized. Through summary and analysis, the main challenges in the research of catalytic hydrogenation of CO2 to higher alcohols were pointed out, and the future research direction in the field was prospected.关键词:carbon dioxide;catalytic hydrogenation;higher alcohols;catalysts;synthesis mechanisms- 63
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:The presence of by-product water produced during the CO2 hydrogenation to methanol reaction will accelerate the aggregation and sintering of CuZn species, resulting in serious deactivation of the catalyst. CeO2 has weak hydrophilicity and high hydrothermal stability, which can enhance the dispersion of CuZn species. Consequently, a series of CuZn-based catalysts with controllable crystal planes of CeO2 carriers were synthesized by hydrothermal method, and appropriate concentrations of oxygen vacancy were strategically introduced. The physicochemical properties such as morphologies and structures and reduction performances of the synthesized CeO2 carriers and CuZn/CeO2-y catalysts (y represents rod、cube or otca) were studied by characterization methods such as TEM, XRD and H2-TPR. The catalytic performances of CuZn/CeO2-y catalysts in CO2 hydrogenation to methanol were also investigated. The results show that the CeO2 carrier with nanorod structure and exposed (110) crystal plane (CeO2-rod) is more conducive to the dispersion of CuZn-based species. Moreover, CeO2-rod and Cu species form Cu—O—Ce interface, which enhances the ability of the catalyst to adsorb and activate CO2 and H2 simultaneously. Therefore, CuZn/CeO2-rod catalyst exhibits high CO2 conversion and methanol selectivity. Under the conditions of 260 °C and 3 MPa, the space-time yield of methanol is up to 433.4 g/(kg·h), and methanol selectivity is up to 68.5%. Simultaneously, the reaction paths and evolution of intermediates in CO2 hydrogenation to methanol were thoroughly delineated by in situ diffuse reflectance infrared Fourier transform spectroscopy. It is found that under the action of CuZn/CeO2 catalysts, the reaction mainly follow the formate path. The crystal plane effect of the carrier does not change the reaction paths, but it increases the equilibrium rate of important intermediate species.关键词:CuZn/CeO2 catalyst;CO2 hydrogenation;methanol selectivity;oxygen vacancy
摘要:The presence of by-product water produced during the CO2 hydrogenation to methanol reaction will accelerate the aggregation and sintering of CuZn species, resulting in serious deactivation of the catalyst. CeO2 has weak hydrophilicity and high hydrothermal stability, which can enhance the dispersion of CuZn species. Consequently, a series of CuZn-based catalysts with controllable crystal planes of CeO2 carriers were synthesized by hydrothermal method, and appropriate concentrations of oxygen vacancy were strategically introduced. The physicochemical properties such as morphologies and structures and reduction performances of the synthesized CeO2 carriers and CuZn/CeO2-y catalysts (y represents rod、cube or otca) were studied by characterization methods such as TEM, XRD and H2-TPR. The catalytic performances of CuZn/CeO2-y catalysts in CO2 hydrogenation to methanol were also investigated. The results show that the CeO2 carrier with nanorod structure and exposed (110) crystal plane (CeO2-rod) is more conducive to the dispersion of CuZn-based species. Moreover, CeO2-rod and Cu species form Cu—O—Ce interface, which enhances the ability of the catalyst to adsorb and activate CO2 and H2 simultaneously. Therefore, CuZn/CeO2-rod catalyst exhibits high CO2 conversion and methanol selectivity. Under the conditions of 260 °C and 3 MPa, the space-time yield of methanol is up to 433.4 g/(kg·h), and methanol selectivity is up to 68.5%. Simultaneously, the reaction paths and evolution of intermediates in CO2 hydrogenation to methanol were thoroughly delineated by in situ diffuse reflectance infrared Fourier transform spectroscopy. It is found that under the action of CuZn/CeO2 catalysts, the reaction mainly follow the formate path. The crystal plane effect of the carrier does not change the reaction paths, but it increases the equilibrium rate of important intermediate species.关键词:CuZn/CeO2 catalyst;CO2 hydrogenation;methanol selectivity;oxygen vacancy- 36
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:The direct synthesis of light olefins through CO2 hydrogenation catalyzed by Fe based catalysts is one of the important ways to achieve the resource utilization of CO2. At present, the catalytic activity and light olefin selectivity of Fe based catalysts for CO2 hydrogenation reaction are still relatively low. A series of Fe based catalysts were prepared by a two-step method of template-equal volume impregnation. The catalytic performance and mechanism of alkali metal promoter Na and transition metal promoter Zn on Fe based catalysts for CO2 hydrogenation to light olefins were studied. The results show that in the CO2 hydrogenation reaction (reaction conditions: t = 320 ℃, p = 2.0 MPa, V(H2):V(CO2) = 3:1, space velocity of 8000 mL/(g·h)), the Fe2Zn1-Na catalyst modified with Zn and Na additives (n(Fe):n(Zn) = 2:1, Na loading (mass fraction) of 2%) shows the optimal catalytic performance (CO2 conversion rate of 36.5%, CO selectivity of 13.6% and C2~C4 olefin and alkane ratio of 6.5). Moreover, after 80 h of online reaction, the catalytic activity and product selectivity of the catalyst remaineds relatively stable. The catalysts before and after the reaction were characterized by N2 adsorption/desorption, XRD, H2-TPR, CO-TPR, CO2-TPD and XPS. The characterization results show that the additive Na can promote the formation and stable existence of Fe5C2 phase in Fe based catalysts during the reaction process. The introduction of Zn as an auxiliary agent resulteds in the formation of ZnFe2O4 phase on Fe based catalysts, which greatly improveds the stability of Fe2Zn-Na catalyst under the simultaneous action of Na. Compared with Fe-Na catalyst, the electron cloud density around Fe species in Fe2Zn1-Na catalyst is stronger, and the light olefin selectivity is higher, but the induction period is slightly longer. The addition of Na and Zn can not only promote the carbonization process of Fe based catalysts, but also enhance the surface alkalinity of the catalysts and promote the adsorption of CO2 on its surface, thereby increasing the conversion rate of CO2.关键词:CO2 hydrogenation;light olefins;ZnFe2O4;promoters;stability
摘要:The direct synthesis of light olefins through CO2 hydrogenation catalyzed by Fe based catalysts is one of the important ways to achieve the resource utilization of CO2. At present, the catalytic activity and light olefin selectivity of Fe based catalysts for CO2 hydrogenation reaction are still relatively low. A series of Fe based catalysts were prepared by a two-step method of template-equal volume impregnation. The catalytic performance and mechanism of alkali metal promoter Na and transition metal promoter Zn on Fe based catalysts for CO2 hydrogenation to light olefins were studied. The results show that in the CO2 hydrogenation reaction (reaction conditions: t = 320 ℃, p = 2.0 MPa, V(H2):V(CO2) = 3:1, space velocity of 8000 mL/(g·h)), the Fe2Zn1-Na catalyst modified with Zn and Na additives (n(Fe):n(Zn) = 2:1, Na loading (mass fraction) of 2%) shows the optimal catalytic performance (CO2 conversion rate of 36.5%, CO selectivity of 13.6% and C2~C4 olefin and alkane ratio of 6.5). Moreover, after 80 h of online reaction, the catalytic activity and product selectivity of the catalyst remaineds relatively stable. The catalysts before and after the reaction were characterized by N2 adsorption/desorption, XRD, H2-TPR, CO-TPR, CO2-TPD and XPS. The characterization results show that the additive Na can promote the formation and stable existence of Fe5C2 phase in Fe based catalysts during the reaction process. The introduction of Zn as an auxiliary agent resulteds in the formation of ZnFe2O4 phase on Fe based catalysts, which greatly improveds the stability of Fe2Zn-Na catalyst under the simultaneous action of Na. Compared with Fe-Na catalyst, the electron cloud density around Fe species in Fe2Zn1-Na catalyst is stronger, and the light olefin selectivity is higher, but the induction period is slightly longer. The addition of Na and Zn can not only promote the carbonization process of Fe based catalysts, but also enhance the surface alkalinity of the catalysts and promote the adsorption of CO2 on its surface, thereby increasing the conversion rate of CO2.关键词:CO2 hydrogenation;light olefins;ZnFe2O4;promoters;stability- 38
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:CO2 hydrogenation to methanol is one of promising route for CO2 utilization. In-based catalysts has high methanol selectivity but low CO2 conversion rate for CO2 hydrogenation to methanol. In order to investigate the effects of doping different proportions of Co and Zr on the performances of In-based catalysts, In-based catalysts doped with different n(Zr):n(Co) were synthesized by co-precipitation method while keeping the same mole fraction of In in the precursor. The catalysts were characterized by low-temperature Ar physical adsorption, X-ray diffraction (XRD), high resolution transmission electron microscope (HRTEM), X-ray photoelectron spectroscopy (XPS) and H2 temperature-programmed reduction (H2-TPR). The catalytic performances of each catalyst were tested under the conditions of temperature from 240 ℃ to 300 ℃, pressure of 3.0 MPa and gas space velocity of 7200 mL/(h·g). The results show that the catalysts with Co and Zr at the same time has a higher CO2 conversion rate and methanol time-space yield than the catalysts with Co or Zr alone in a certain n(Zr):n(Co) range. n(Zr):n(Co) will produce different degrees of metal-to-metal interactions, which will affect the specific surface area, particle size and reduction performance of the catalysts. When the doped n(Zr):n(Co) is 1:3, the catalyst has the best methanol synthesis ability and the methanol time-space yield is up to 178 mg/(g·h). The CO2 conversion rate decreases in the order: Zr5Co15In, Zr2.5Co17.5In, Zr7.5Co12.5In, Co20In, Zr10Co10In, Zr20In and In100, which is consistent with the trend of oxygen vacancy ratio. The Zr5Co15In catalyst has a smaller particle size with a larger specific surface area, which can expose more active sites, and has higher reducibility and stronger intermetallic interactions. The CO2 conversion rate of Zr5Co15In can reach 13.63%, which is 19.9% higher than Co20In and 64.7% higher than Zr20In.关键词:methanol;In-based catalysts;carbon dioxide hydrogenation;co-precipitation
摘要:CO2 hydrogenation to methanol is one of promising route for CO2 utilization. In-based catalysts has high methanol selectivity but low CO2 conversion rate for CO2 hydrogenation to methanol. In order to investigate the effects of doping different proportions of Co and Zr on the performances of In-based catalysts, In-based catalysts doped with different n(Zr):n(Co) were synthesized by co-precipitation method while keeping the same mole fraction of In in the precursor. The catalysts were characterized by low-temperature Ar physical adsorption, X-ray diffraction (XRD), high resolution transmission electron microscope (HRTEM), X-ray photoelectron spectroscopy (XPS) and H2 temperature-programmed reduction (H2-TPR). The catalytic performances of each catalyst were tested under the conditions of temperature from 240 ℃ to 300 ℃, pressure of 3.0 MPa and gas space velocity of 7200 mL/(h·g). The results show that the catalysts with Co and Zr at the same time has a higher CO2 conversion rate and methanol time-space yield than the catalysts with Co or Zr alone in a certain n(Zr):n(Co) range. n(Zr):n(Co) will produce different degrees of metal-to-metal interactions, which will affect the specific surface area, particle size and reduction performance of the catalysts. When the doped n(Zr):n(Co) is 1:3, the catalyst has the best methanol synthesis ability and the methanol time-space yield is up to 178 mg/(g·h). The CO2 conversion rate decreases in the order: Zr5Co15In, Zr2.5Co17.5In, Zr7.5Co12.5In, Co20In, Zr10Co10In, Zr20In and In100, which is consistent with the trend of oxygen vacancy ratio. The Zr5Co15In catalyst has a smaller particle size with a larger specific surface area, which can expose more active sites, and has higher reducibility and stronger intermetallic interactions. The CO2 conversion rate of Zr5Co15In can reach 13.63%, which is 19.9% higher than Co20In and 64.7% higher than Zr20In.关键词:methanol;In-based catalysts;carbon dioxide hydrogenation;co-precipitation- 45
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:Hydrogenation of CO2 to synthesize CH4 (CO2 methanation) is one of the crucial pathways for the efficient and clean conversion of CO2. Despite extensive researches on the catalytic system for CO2 methanation, the intrinsic kinetics of CO2 methanation under specific conditions have not been thoroughly investigated. Ni/CeO2 catalyst (Ni mass fraction of 10%) was prepared using the combustion method, and samples with varying m(α-Al2O3):m(Ni/CeO2) were produced by incorporating α-Al2O3 as the internal diluent. The intrinsic kinetics of CO2 methanation of sample A with m(α-Al2O3):m(Ni/CeO2) = 25:1 were examined under the conditions of temperature of 300 ℃, pressure of 1 MPa and gas hourly space velocity of 3 × 106 mL/(g·h). An in-depth analysis of the kinetic equations for the intrinsic CO2 methanation reaction was conducted through a combination of intrinsic kinetic tests, diffuse reflection infrared spectrum, H2-temperature programmed reaction and H2-temperature programmed desorption, etc. The results show that the CH4 generation rate under action of sample A can reach up to 41.4 mmol/(g·h)(H2 partial pressure is 400 kPa, and CO2 partial pressure is 120 kPa). When CO2 partial pressure is 30 kPa to 120 kPa, the CH4 generation rate exhibits linear increases with the rises of the H2 partial pressure, and is not affected by variation of the CO2 or CO partial pressure. Within this CO2 partial pressure range, the hydrogen-rich environment significantly enhances the catalytic activity of the catalyst for CO2 methanation.关键词:CO2 methanation;Ni/CeO2 catalyst;intrinsic dynamics
摘要:Hydrogenation of CO2 to synthesize CH4 (CO2 methanation) is one of the crucial pathways for the efficient and clean conversion of CO2. Despite extensive researches on the catalytic system for CO2 methanation, the intrinsic kinetics of CO2 methanation under specific conditions have not been thoroughly investigated. Ni/CeO2 catalyst (Ni mass fraction of 10%) was prepared using the combustion method, and samples with varying m(α-Al2O3):m(Ni/CeO2) were produced by incorporating α-Al2O3 as the internal diluent. The intrinsic kinetics of CO2 methanation of sample A with m(α-Al2O3):m(Ni/CeO2) = 25:1 were examined under the conditions of temperature of 300 ℃, pressure of 1 MPa and gas hourly space velocity of 3 × 106 mL/(g·h). An in-depth analysis of the kinetic equations for the intrinsic CO2 methanation reaction was conducted through a combination of intrinsic kinetic tests, diffuse reflection infrared spectrum, H2-temperature programmed reaction and H2-temperature programmed desorption, etc. The results show that the CH4 generation rate under action of sample A can reach up to 41.4 mmol/(g·h)(H2 partial pressure is 400 kPa, and CO2 partial pressure is 120 kPa). When CO2 partial pressure is 30 kPa to 120 kPa, the CH4 generation rate exhibits linear increases with the rises of the H2 partial pressure, and is not affected by variation of the CO2 or CO partial pressure. Within this CO2 partial pressure range, the hydrogen-rich environment significantly enhances the catalytic activity of the catalyst for CO2 methanation.关键词:CO2 methanation;Ni/CeO2 catalyst;intrinsic dynamics- 38
- |
- 0
- |
- 0
发布时间:2024-09-02 -
 摘要:Using deactivated methanol to olefin (MTO) catalyst as a partial aluminum and phosphorus source and entire silicon source, and triethylamine as a template, a directing agent was prepared at low temperature (160 ℃). S-SAPO-34 molecular sieve was then green synthesized by directing agent method, and C-SAPO-34 molecular sieve was synthesized without directing agents. The crystal structures, morphologies, pore structures, acidic characteristics, coordination states and thermal stabilities of the two molecular sieves were analyzed by XRD, SEM, N2 adsorption/desorption, NH3-TPD, solid-state MAS-NMR and TGA. The results indicate that SAPO-34 molecular sieve with high crystallinity can be synthesized by directing agent method under the condition of reducing the amount of organic template agent by 2/3. The catalytic performance evaluation of S-SAPO-34 and C-SAPO-34 molecular sieves for MTO reaction was carried out on a fixed bed reactor under the reaction conditions of temperature 460 ℃, atmospheric pressure, methanol mass space velocity 6.0 h-1 and pure methanol as the raw material. The results show that compared to C-SAPO-34, the crystallization performance of S-SAPO-34 molecular sieve is much better, with a relative crystallinity of 136%, and the crystal morphology is more complete. In MTO reaction, S-SAPO-34 and C-SAPO-34 molecular sieves can ensure methanol conversion, and the highest selectivities of diene (ethylene + propylene) are 82.1% and 82.3%, respectively. This method can provide a new idea for the green synthesis of SAPO-34 molecular sieve under low dosage template conditions.关键词:SAPO-34;deactivated catalyst;directing agent method;low template;MTO reaction
摘要:Using deactivated methanol to olefin (MTO) catalyst as a partial aluminum and phosphorus source and entire silicon source, and triethylamine as a template, a directing agent was prepared at low temperature (160 ℃). S-SAPO-34 molecular sieve was then green synthesized by directing agent method, and C-SAPO-34 molecular sieve was synthesized without directing agents. The crystal structures, morphologies, pore structures, acidic characteristics, coordination states and thermal stabilities of the two molecular sieves were analyzed by XRD, SEM, N2 adsorption/desorption, NH3-TPD, solid-state MAS-NMR and TGA. The results indicate that SAPO-34 molecular sieve with high crystallinity can be synthesized by directing agent method under the condition of reducing the amount of organic template agent by 2/3. The catalytic performance evaluation of S-SAPO-34 and C-SAPO-34 molecular sieves for MTO reaction was carried out on a fixed bed reactor under the reaction conditions of temperature 460 ℃, atmospheric pressure, methanol mass space velocity 6.0 h-1 and pure methanol as the raw material. The results show that compared to C-SAPO-34, the crystallization performance of S-SAPO-34 molecular sieve is much better, with a relative crystallinity of 136%, and the crystal morphology is more complete. In MTO reaction, S-SAPO-34 and C-SAPO-34 molecular sieves can ensure methanol conversion, and the highest selectivities of diene (ethylene + propylene) are 82.1% and 82.3%, respectively. This method can provide a new idea for the green synthesis of SAPO-34 molecular sieve under low dosage template conditions.关键词:SAPO-34;deactivated catalyst;directing agent method;low template;MTO reaction- 21
- |
- 0
- |
- 0
发布时间:2024-09-02 - 摘要:Methanol is a key product connecting modern coal chemical industry and traditional coal chemical industry. Industrially, methanol is typically produced by the reaction of syngas under the action of the catalyst. The service life of methanol synthesis catalysts is usually 3 to 4 years, and ensuring the normal service life of methanol synthesis catalysts is of great significance to methanol enterprises. Typical cases of sulfur poisoning and iron poisoning in industrial methanol synthesis catalysts were analyzed, and the poisoning mechanisms were explained based on literature data. It is pointed out that iron poisoning and sulfur poisoning are the main causes of methanol synthesis catalyst deactivation. Corresponding countermeasures were provided, which can offer guidance for ensuring the normal service life of methanol synthesis catalysts.关键词:methanol;catalysts;deactivation;iron poisoning;sulfur poisoning
- 14
- |
- 0
- |
- 0
发布时间:2024-09-02
0

