
最新刊期
卷 50 , 期 7 , 2025
-
 摘要:A large amount of carbon dioxide emissions caused by the use of fossil fuels have caused a series of environmental problems. Therefore, the thermal catalytic conversion of CO2 to methanol by CuZnAl catalyst is a effective way to reduce CO2 emission, but the catalytic performance of the catalyst still needs to be improved. CZACex catalysts with different Ce doping amounts (mass fraction) were prepared by in situ co-precipitation method, and CZA-5%Ce was prepared by impregnation method. The effects of different Ce doping methods on the physicochemical properties of the catalysts were studied. The physicochemical of the catalyst and the mechanism of Ce were investigated by XRD, N2 adsorption/desorption, H2-TPR and XPS. The results show that Ce doping can provide abundant oxygen vacancies, which can effectively promote CO2 conversion and improve CO2 conversion rate. Different doping methods lead to significant differences in the internal texture properties and Ce interaction forms of catalysts. Doping Ce by impregnation method can enhance the interaction between Ce species and Cu species, which is more conducive to the formation of oxygen vacancies and Ce3+, so that CZA-5%Ce has the best catalytic performance. Under the conditions of 250 ℃, 3 MPa and V(H2):V(CO2) = 3:1, the CO2 conversion rate, methanol selectivity and methanol space-time yield of CZA-5%Ce is 15.63%, 36.35% and 83.35 mg/(mL·h), respectively.关键词:CO2 hydrogenation;methanol;CuZnAl catalyst;Ce doping;oxygen vacancies141|0|0更新时间:2025-07-30
摘要:A large amount of carbon dioxide emissions caused by the use of fossil fuels have caused a series of environmental problems. Therefore, the thermal catalytic conversion of CO2 to methanol by CuZnAl catalyst is a effective way to reduce CO2 emission, but the catalytic performance of the catalyst still needs to be improved. CZACex catalysts with different Ce doping amounts (mass fraction) were prepared by in situ co-precipitation method, and CZA-5%Ce was prepared by impregnation method. The effects of different Ce doping methods on the physicochemical properties of the catalysts were studied. The physicochemical of the catalyst and the mechanism of Ce were investigated by XRD, N2 adsorption/desorption, H2-TPR and XPS. The results show that Ce doping can provide abundant oxygen vacancies, which can effectively promote CO2 conversion and improve CO2 conversion rate. Different doping methods lead to significant differences in the internal texture properties and Ce interaction forms of catalysts. Doping Ce by impregnation method can enhance the interaction between Ce species and Cu species, which is more conducive to the formation of oxygen vacancies and Ce3+, so that CZA-5%Ce has the best catalytic performance. Under the conditions of 250 ℃, 3 MPa and V(H2):V(CO2) = 3:1, the CO2 conversion rate, methanol selectivity and methanol space-time yield of CZA-5%Ce is 15.63%, 36.35% and 83.35 mg/(mL·h), respectively.关键词:CO2 hydrogenation;methanol;CuZnAl catalyst;Ce doping;oxygen vacancies141|0|0更新时间:2025-07-30 -
 摘要:Designing effective inorganic metal catalysts to improve the efficiency of syngas-to-alcohol conversion and the selectivity of higher alcohols (C5+ alcohols) is critical for the development of sustainable chemistry and energy. To investigate the effect of Al additions on the structure and catalytic performance of CoMn catalysts in synthesis of higher alcohols from syngas, CoMnAl catalysts with varying Al addition quantities were produced by co-precipitation. The effects of Al doped on the structure, composition, morphology, valence state and surface chemical properties of CoMn catalysts were compared using characterization techniques such as XRD, SEM, EDX, N2 adsorption/desorption, ICP, XPS and CO/H2/NH3-TPD. The catalytic performance of CoMnAl catalysts was tested under conditions of 260 ℃, 3.0 MPa, V(CO):V(H2):V(Ar) = 80:40:30 and space velocity of 3000 h-1. The results show that when Al is doped in appropriate amounts as a structural promoter, it interacts with other metals to variable degrees, influencing the specific surface area and particle size of catalyst. As an electronic promoter, Al facilitates electron transfer with Co to form a Co-Al-O active phase, which in turn affects the reducibility and formation of oxygen defects of the catalyst, significantly enhancing its catalytic performance. In situ FT-IR characterization reveals that the reaction pathway of the CoMnAl(0.3) catalyst doped with Al additives is an aldehyde insertion process, which effectively inhibits the formation of methane in the reaction and improves the total alcohol selectivity to 42%. The percentage of higher alcohols in the total alcohols reaches 48.8% (mole fraction). By precisely controlling the Al doping amount, it is possible to effectively regulate the structure and surface oxygen defects of the catalyst, thereby enhancing the selectivity for higher alcohols in the syngas-to-alcohol process.关键词:syngas;higher alcohols;Al additives;oxygen defects75|0|0更新时间:2025-07-30
摘要:Designing effective inorganic metal catalysts to improve the efficiency of syngas-to-alcohol conversion and the selectivity of higher alcohols (C5+ alcohols) is critical for the development of sustainable chemistry and energy. To investigate the effect of Al additions on the structure and catalytic performance of CoMn catalysts in synthesis of higher alcohols from syngas, CoMnAl catalysts with varying Al addition quantities were produced by co-precipitation. The effects of Al doped on the structure, composition, morphology, valence state and surface chemical properties of CoMn catalysts were compared using characterization techniques such as XRD, SEM, EDX, N2 adsorption/desorption, ICP, XPS and CO/H2/NH3-TPD. The catalytic performance of CoMnAl catalysts was tested under conditions of 260 ℃, 3.0 MPa, V(CO):V(H2):V(Ar) = 80:40:30 and space velocity of 3000 h-1. The results show that when Al is doped in appropriate amounts as a structural promoter, it interacts with other metals to variable degrees, influencing the specific surface area and particle size of catalyst. As an electronic promoter, Al facilitates electron transfer with Co to form a Co-Al-O active phase, which in turn affects the reducibility and formation of oxygen defects of the catalyst, significantly enhancing its catalytic performance. In situ FT-IR characterization reveals that the reaction pathway of the CoMnAl(0.3) catalyst doped with Al additives is an aldehyde insertion process, which effectively inhibits the formation of methane in the reaction and improves the total alcohol selectivity to 42%. The percentage of higher alcohols in the total alcohols reaches 48.8% (mole fraction). By precisely controlling the Al doping amount, it is possible to effectively regulate the structure and surface oxygen defects of the catalyst, thereby enhancing the selectivity for higher alcohols in the syngas-to-alcohol process.关键词:syngas;higher alcohols;Al additives;oxygen defects75|0|0更新时间:2025-07-30 -
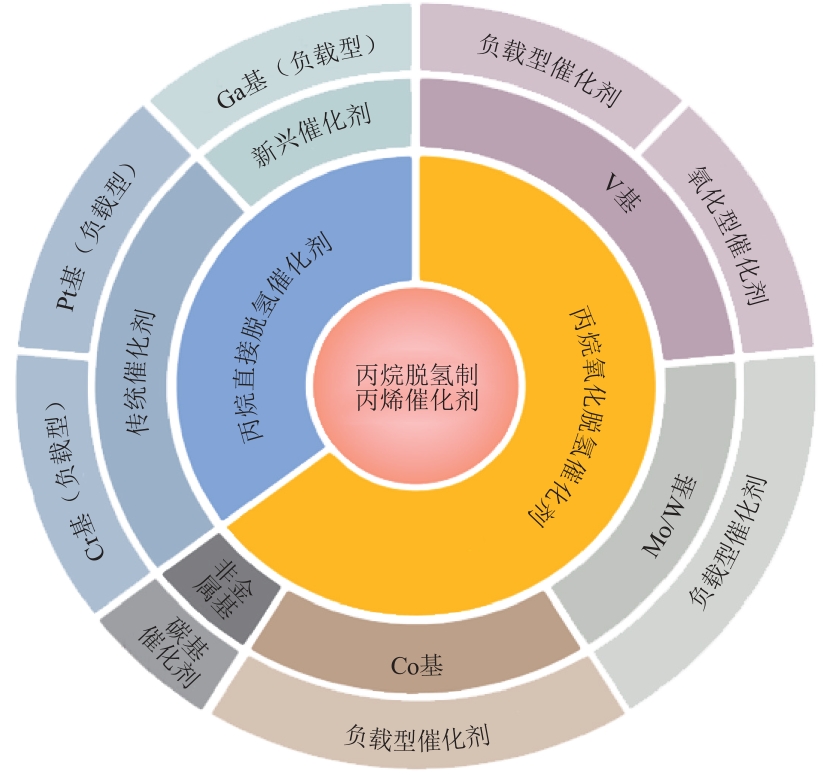 摘要:Propane dehydrogenation to propylene is one of the important sources of propylene supply. The factors affecting the performance and catalytic mechanism of two kinds of catalysts for propane dehydrogenation to propylene were reviewed. The problems of carrier screening, auxiliary optimization, interaction between carrier and active component and deactivation of various catalysts in recent years were summarized and analyzed. In the process of oxidative dehydrogenation of propane, the V-based catalyst is environmentally friendly, but the deactivation is fast and the activation temperature is high. The activity problem of Mo/W-based catalyst should be solved and the catalytic mechanism should be clarified. The reaction condition of Co-based catalyst is mild, but the process condition suitable for industrial production should be explored. The C-based catalyst should further verify the high temperature tolerance of carbon support and further increase the number and dispersion of functional groups on carbon support. In the process of direct dehydrogenation of propane, the Cr-based catalyst should reduce Cr content while maintaining activity and seek alternative active components. The Pt-based catalyst should increase the dispersion of Pt particles and minimize coking amount. When increasing the activity of Ga-based catalyst, the stable existence time of active specie should be prolonged as much as possible.关键词:propane dehydrogenation;propylene;catalyst196|0|0更新时间:2025-07-30
摘要:Propane dehydrogenation to propylene is one of the important sources of propylene supply. The factors affecting the performance and catalytic mechanism of two kinds of catalysts for propane dehydrogenation to propylene were reviewed. The problems of carrier screening, auxiliary optimization, interaction between carrier and active component and deactivation of various catalysts in recent years were summarized and analyzed. In the process of oxidative dehydrogenation of propane, the V-based catalyst is environmentally friendly, but the deactivation is fast and the activation temperature is high. The activity problem of Mo/W-based catalyst should be solved and the catalytic mechanism should be clarified. The reaction condition of Co-based catalyst is mild, but the process condition suitable for industrial production should be explored. The C-based catalyst should further verify the high temperature tolerance of carbon support and further increase the number and dispersion of functional groups on carbon support. In the process of direct dehydrogenation of propane, the Cr-based catalyst should reduce Cr content while maintaining activity and seek alternative active components. The Pt-based catalyst should increase the dispersion of Pt particles and minimize coking amount. When increasing the activity of Ga-based catalyst, the stable existence time of active specie should be prolonged as much as possible.关键词:propane dehydrogenation;propylene;catalyst196|0|0更新时间:2025-07-30 -
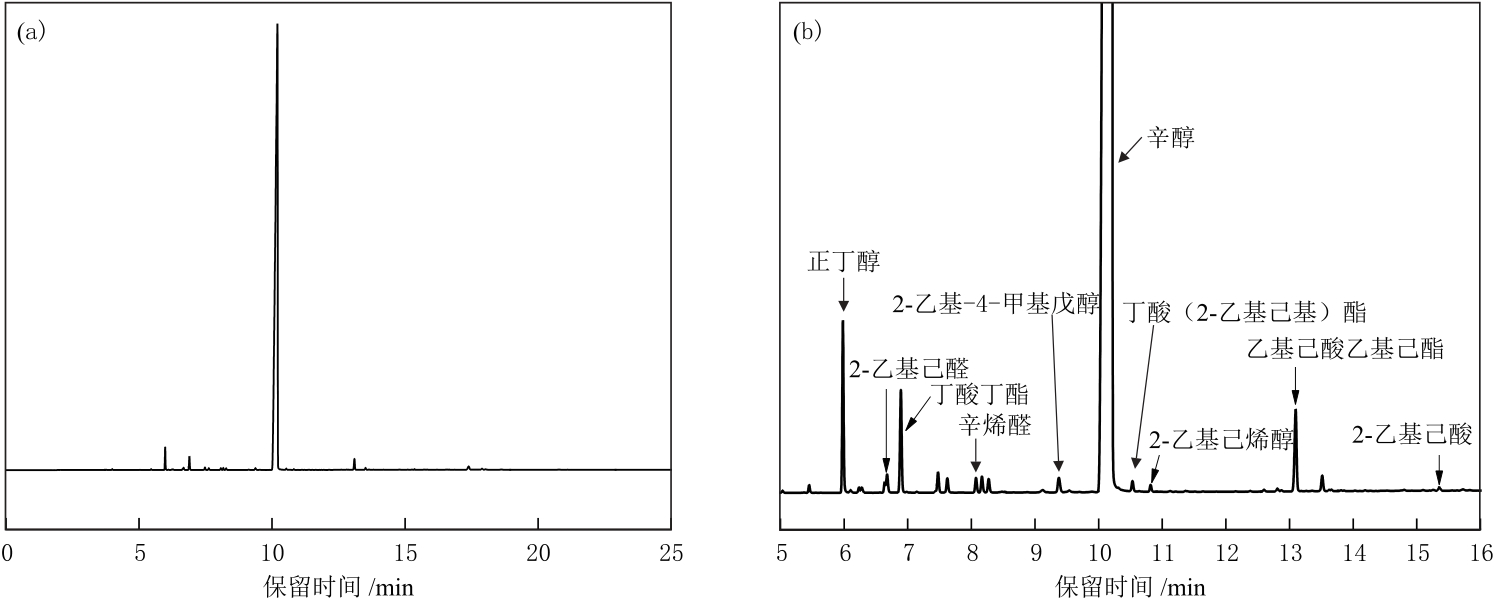 摘要:Gas phase hydrogenation of 2-ethyl-2-hexenal is the main method for industrial production of 2-ethylhexanol (hereinafter referred to as “octanol”). The type and selectivity of by-products in the process have direct impacts on the quality and yield of octanol. To achieve a highly active catalyst and control the selectivity of by-products, copper (Cu)-based catalysts were prepared and modified with SiO2 as the promoter. The catalytic performances of obtained catalysts (N-1, N-2 and N-3) for gas phase hydrogenation of 2-ethyl-2-hexenal were measured and the structures of the catalysts were analyzed by XRD, N2 adsorption/desorption, XPS, TEM, NH3-TPD, Py-IR, etc. The results show that the hydrogenation activities of the catalysts are related to the dispersions of CuO and the amounts of Lewis acid in the catalysts. Under the reaction conditions of inlet temperature of 150 ℃, pressure of 0.4 MPa and 2-ethyl-2-hexenal liquid space velocity of 0.3 h-1, 2-ethyl-2-hexenal conversion rate of 99.76% and octanol selectivity of 99.50% are achieved for N-1 with SiO2 content (mass fraction, the same below) of 0. The contents of partially hydrogenated by-products 2-ethylhexanal and 2-ethylhexenol in reaction products are 0.22% and 0.04%, respectively, and the content of heavy components is 1.06%. Modification of catalyst surface properties with SiO2 can regulate the selectivity of heavy components. N-2 with SiO2 content of 2% exhibits 2-ethyl-2-hexenal conversion rate of 99.49% and octanol selectivity of 99.40%, with the content of 2-ethylhexanal, 2-ethylhexenol and heavy components of 0.28%, 0.06% and 0.72%, respectively. During a total of ten months of industrial application verification, N-2 demonstrates superior catalytic performance with average contents of 2-ethylhexanal, octanol and heavy components of less than 0.01%, 97.88% and 0.42%, respectively.关键词:2-ethyl-2-hexenal hydrogenation;Cu-based catalyst;2-ethylhexanol;SiO2;by-products selectivity233|0|0更新时间:2025-07-30
摘要:Gas phase hydrogenation of 2-ethyl-2-hexenal is the main method for industrial production of 2-ethylhexanol (hereinafter referred to as “octanol”). The type and selectivity of by-products in the process have direct impacts on the quality and yield of octanol. To achieve a highly active catalyst and control the selectivity of by-products, copper (Cu)-based catalysts were prepared and modified with SiO2 as the promoter. The catalytic performances of obtained catalysts (N-1, N-2 and N-3) for gas phase hydrogenation of 2-ethyl-2-hexenal were measured and the structures of the catalysts were analyzed by XRD, N2 adsorption/desorption, XPS, TEM, NH3-TPD, Py-IR, etc. The results show that the hydrogenation activities of the catalysts are related to the dispersions of CuO and the amounts of Lewis acid in the catalysts. Under the reaction conditions of inlet temperature of 150 ℃, pressure of 0.4 MPa and 2-ethyl-2-hexenal liquid space velocity of 0.3 h-1, 2-ethyl-2-hexenal conversion rate of 99.76% and octanol selectivity of 99.50% are achieved for N-1 with SiO2 content (mass fraction, the same below) of 0. The contents of partially hydrogenated by-products 2-ethylhexanal and 2-ethylhexenol in reaction products are 0.22% and 0.04%, respectively, and the content of heavy components is 1.06%. Modification of catalyst surface properties with SiO2 can regulate the selectivity of heavy components. N-2 with SiO2 content of 2% exhibits 2-ethyl-2-hexenal conversion rate of 99.49% and octanol selectivity of 99.40%, with the content of 2-ethylhexanal, 2-ethylhexenol and heavy components of 0.28%, 0.06% and 0.72%, respectively. During a total of ten months of industrial application verification, N-2 demonstrates superior catalytic performance with average contents of 2-ethylhexanal, octanol and heavy components of less than 0.01%, 97.88% and 0.42%, respectively.关键词:2-ethyl-2-hexenal hydrogenation;Cu-based catalyst;2-ethylhexanol;SiO2;by-products selectivity233|0|0更新时间:2025-07-30 -
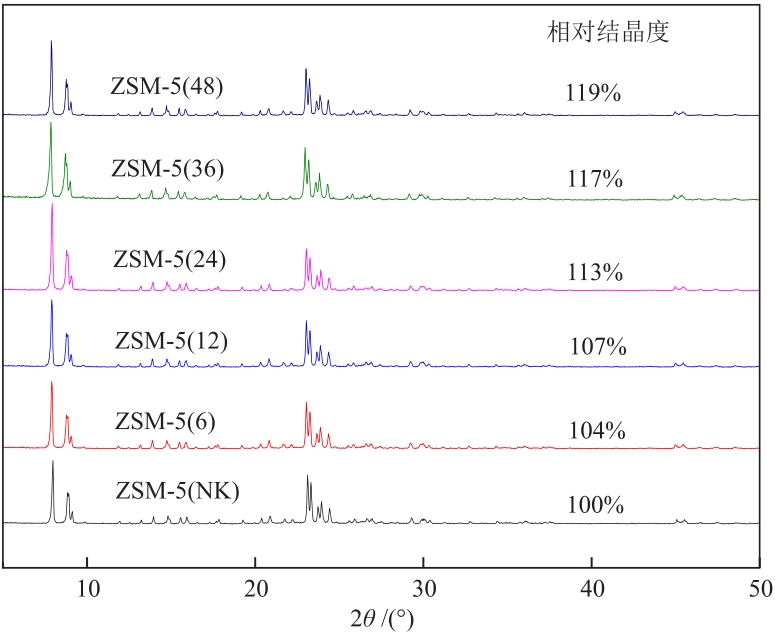 摘要:In order to improve the anti-coking performance of the conventional zeolite catalyst, a series of Zn/ZSM-5 catalysts (Zn/ZSM-5(t), t is the secondary crystallization time) with Zn mass fraction of 2% were prepared by the secondary crystallization treatment of ZSM-5 using tetrapropylammonium hydroxide (TPAOH). Zn/ZSM-5(t) were characterized by XRD, TEM, N2 absorption/desorption, etc. And the catalytic performances of the catalysts were investigated. The results show that during the desilication and recrystallization process, Si species inside ZSM-5 can be removed by OH-, and then recrystallize on the surface of ZSM-5 under the action of tetrapropylammonium cation (TPA+), so that the catalysts form a hollow structure. With the increase of secondary crystallization time, the relative crystallinities, mesoporous volumes and shell thicknesses of Zn/ZSM-5(t) increase gradually, while the acid strengths and acid amounts of the catalysts decrease gradually. Under the conditions of propane flow rate of 5 mL/min and temperature of 600 ℃ for 4 h, Zn/ZSM-5(36) exhibits the best catalytic performance with propane conversion rate and aromatic selectivity (such as benzene, methylbenzene) of 80.0% and 70.6%, respectively. In addition, Zn/ZSM-5(36) has good anti-carbon deposition performance with carbon deposition amount of 1.8% after reaction for 4 h.关键词:hollow structure;secondary crystallization;propane aromatization;Zn/ZSM-5 catalysts181|0|0更新时间:2025-07-30
摘要:In order to improve the anti-coking performance of the conventional zeolite catalyst, a series of Zn/ZSM-5 catalysts (Zn/ZSM-5(t), t is the secondary crystallization time) with Zn mass fraction of 2% were prepared by the secondary crystallization treatment of ZSM-5 using tetrapropylammonium hydroxide (TPAOH). Zn/ZSM-5(t) were characterized by XRD, TEM, N2 absorption/desorption, etc. And the catalytic performances of the catalysts were investigated. The results show that during the desilication and recrystallization process, Si species inside ZSM-5 can be removed by OH-, and then recrystallize on the surface of ZSM-5 under the action of tetrapropylammonium cation (TPA+), so that the catalysts form a hollow structure. With the increase of secondary crystallization time, the relative crystallinities, mesoporous volumes and shell thicknesses of Zn/ZSM-5(t) increase gradually, while the acid strengths and acid amounts of the catalysts decrease gradually. Under the conditions of propane flow rate of 5 mL/min and temperature of 600 ℃ for 4 h, Zn/ZSM-5(36) exhibits the best catalytic performance with propane conversion rate and aromatic selectivity (such as benzene, methylbenzene) of 80.0% and 70.6%, respectively. In addition, Zn/ZSM-5(36) has good anti-carbon deposition performance with carbon deposition amount of 1.8% after reaction for 4 h.关键词:hollow structure;secondary crystallization;propane aromatization;Zn/ZSM-5 catalysts181|0|0更新时间:2025-07-30 -
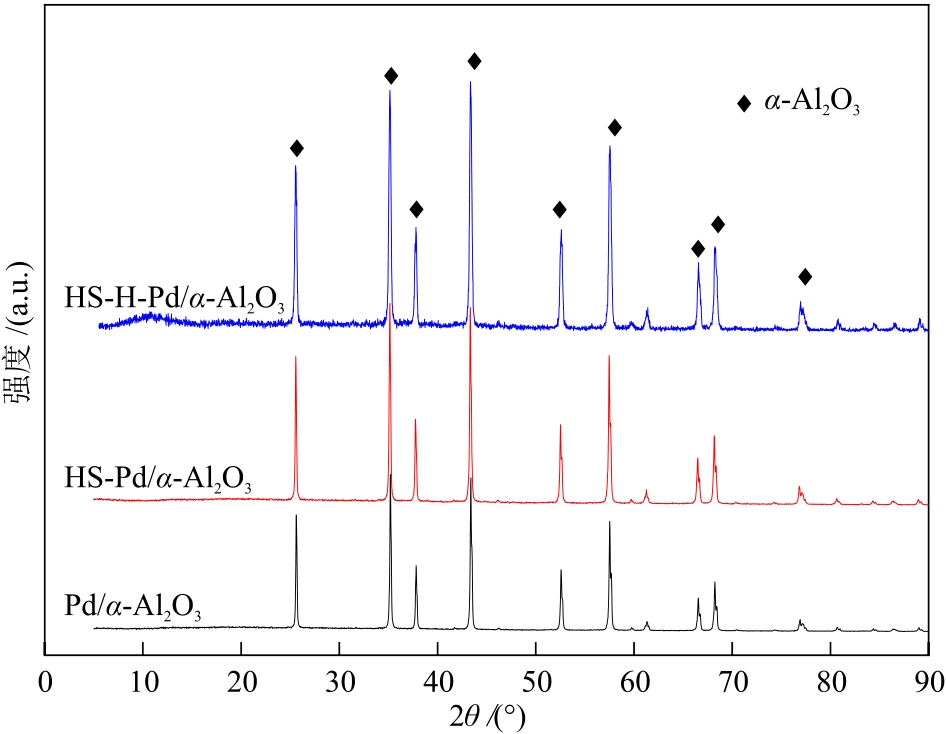 摘要:Dimethyl oxalate (DMO) is an intermediate product of syngas to ethylene glycol. In industry, DMO is typically produced via the reaction of CO and methyl nitrite (MN) over Pd/α-Al2O3 catalyst. However, during the production of DMO using industrial tail gas, significant load fluctuations are unavoidable, and the concentration of toxic substances such as H2S may exceed permissible limits. Therefore, investigating the toxic effects of H2S on the catalyst is of critical importance. The impact of H2S on catalytic performance of the Pd/α-Al2O3 catalyst for synthesis of DMO from CO and MN was examined using a fixed-bed microreactor. Under the temperature of 120 ℃, normal pressure, n(CO)/n(MN) of 2.0, and the space velocity of 3000 h-1, after exposure to H2S poisoning, the conversion rate of MN decreases from 83% to about 64%. After H2 reduction treatment on the poisoned catalyst, the MN conversion rate further decreases to about 51%. The structures of catalysts were characterized by XRD, FT-IR, HRTEM and XPS, etc. The results indicate that H2S preferentially reacts with palladium active species on the catalyst surface to form inert compounds such as PdSO4, PdOx-S, and PdOx-SOx, while the palladium particles retain Pd0 state internally. H2 reduction treatment removes some surface sulfur species and some oxidized Pd species are reduced back to Pd0. However, this reduction process induces the aggregation of Pd0 particles. The occupation and coverage of active sites by inert species such as PdSO4, PdOx-S, and PdOx-SOx, along with the aggregation of Pd0 are the main reasons for the decrease of cataltic activity of catalyst.关键词:dimethyl oxalate;Pd/α-Al2O3 catalyst;H2S pretreatment;regenerative treatment11|0|0更新时间:2025-07-30
摘要:Dimethyl oxalate (DMO) is an intermediate product of syngas to ethylene glycol. In industry, DMO is typically produced via the reaction of CO and methyl nitrite (MN) over Pd/α-Al2O3 catalyst. However, during the production of DMO using industrial tail gas, significant load fluctuations are unavoidable, and the concentration of toxic substances such as H2S may exceed permissible limits. Therefore, investigating the toxic effects of H2S on the catalyst is of critical importance. The impact of H2S on catalytic performance of the Pd/α-Al2O3 catalyst for synthesis of DMO from CO and MN was examined using a fixed-bed microreactor. Under the temperature of 120 ℃, normal pressure, n(CO)/n(MN) of 2.0, and the space velocity of 3000 h-1, after exposure to H2S poisoning, the conversion rate of MN decreases from 83% to about 64%. After H2 reduction treatment on the poisoned catalyst, the MN conversion rate further decreases to about 51%. The structures of catalysts were characterized by XRD, FT-IR, HRTEM and XPS, etc. The results indicate that H2S preferentially reacts with palladium active species on the catalyst surface to form inert compounds such as PdSO4, PdOx-S, and PdOx-SOx, while the palladium particles retain Pd0 state internally. H2 reduction treatment removes some surface sulfur species and some oxidized Pd species are reduced back to Pd0. However, this reduction process induces the aggregation of Pd0 particles. The occupation and coverage of active sites by inert species such as PdSO4, PdOx-S, and PdOx-SOx, along with the aggregation of Pd0 are the main reasons for the decrease of cataltic activity of catalyst.关键词:dimethyl oxalate;Pd/α-Al2O3 catalyst;H2S pretreatment;regenerative treatment11|0|0更新时间:2025-07-30 -
 摘要:With the increase of human activities and the acceleration of industrializatio, climate change problems caused by greenhouse gas emissions, including global warming, are becoming increasingly serious. Fluorinated greenhouse gases have extremely high global warming potentials (GWP), causing serious greenhouse effects, and have been strictly regulated by international conventions. The research progress on membrane separation technologies and membrane materials for various fluorinated greenhouse gases (SF6, NF3, perfluocarbon (PFCs) and trifluoromethane (R23), etc.) was reviewed, with a focus on analyzing the selectivity, permeability and stability of different membrane materials. The feasibility of improving the recovery rate and purity of fluorinated greenhouse gases by optimizing membrane component structure and process parameters was explored. Developing new membrane materials with high selectivity and stability, optimizing membrane module design and exploring separation methods for complex gases are the research directions for high-performance membrane materials.关键词:greenhouse gas;SF6;NF3;perfluocarbon;trifluoromethane171|0|0更新时间:2025-07-30
摘要:With the increase of human activities and the acceleration of industrializatio, climate change problems caused by greenhouse gas emissions, including global warming, are becoming increasingly serious. Fluorinated greenhouse gases have extremely high global warming potentials (GWP), causing serious greenhouse effects, and have been strictly regulated by international conventions. The research progress on membrane separation technologies and membrane materials for various fluorinated greenhouse gases (SF6, NF3, perfluocarbon (PFCs) and trifluoromethane (R23), etc.) was reviewed, with a focus on analyzing the selectivity, permeability and stability of different membrane materials. The feasibility of improving the recovery rate and purity of fluorinated greenhouse gases by optimizing membrane component structure and process parameters was explored. Developing new membrane materials with high selectivity and stability, optimizing membrane module design and exploring separation methods for complex gases are the research directions for high-performance membrane materials.关键词:greenhouse gas;SF6;NF3;perfluocarbon;trifluoromethane171|0|0更新时间:2025-07-30 -
 摘要:The enrichment of CH4 in low-concentration coalbed methane requires high-performance CH4/N2 adsorbents. T-zeolite was successfully synthesized using a hydrothermal method and characterized by PXRD, SEM, and N2 adsorption/desorption. The CH4 and N2 single-component adsorption performance of T-zeolite was tested, and its Ideal Adsorbed Solution Theory (IAST) selectivity and adsorption heat were calculated. The CH4/N2 separation performance of T-zeolite was also investigated. The results show that at 298 K and 0.1 MPa, the CH4 adsorption capacity of T-zeolite (Tz-168) is 21.4 cm3/g, and the CH4/N2 selectivity of T-zeolite is 5.5, which is better than most molecular sieve adsorbents. CH4/N2 mixture permeation experiments further confirm that T-zeolite exhibits excellent CH4/N2 separation performance, with actual separation time of 5 min and 7 min at V(CH4):V(N2) of 50:50 and 20:80, respectively. This has significant application potential in the enrichment of CH4 from low-concentration coalbed methane.关键词:coalbed methane;T-zeolite;CH4/N2;adsorption separation36|0|0更新时间:2025-07-30
摘要:The enrichment of CH4 in low-concentration coalbed methane requires high-performance CH4/N2 adsorbents. T-zeolite was successfully synthesized using a hydrothermal method and characterized by PXRD, SEM, and N2 adsorption/desorption. The CH4 and N2 single-component adsorption performance of T-zeolite was tested, and its Ideal Adsorbed Solution Theory (IAST) selectivity and adsorption heat were calculated. The CH4/N2 separation performance of T-zeolite was also investigated. The results show that at 298 K and 0.1 MPa, the CH4 adsorption capacity of T-zeolite (Tz-168) is 21.4 cm3/g, and the CH4/N2 selectivity of T-zeolite is 5.5, which is better than most molecular sieve adsorbents. CH4/N2 mixture permeation experiments further confirm that T-zeolite exhibits excellent CH4/N2 separation performance, with actual separation time of 5 min and 7 min at V(CH4):V(N2) of 50:50 and 20:80, respectively. This has significant application potential in the enrichment of CH4 from low-concentration coalbed methane.关键词:coalbed methane;T-zeolite;CH4/N2;adsorption separation36|0|0更新时间:2025-07-30 -
 摘要:The application of pressure swing adsorption (PSA) to extract CO₂ from the exhaust gas of lithium ore roasting (natural gas) furnaces and employ it as a carbonization feedstock in the production of lithium carbonate from spodumene has garnered extensive interest. Considering the low CO2 concentration and elevated temperature characteristics of the exhaust gas, a two-stage vacuum pressure swing adsorption (VPSA) process was specifically designed, and the process was simulated using Aspen Adsorption software. The results show that using 13X zeolite as the adsorbent under feed gas (roasting kiln exhaust gas) temperature of 373.15 K, regeneration pressure of 5 kPa, and CO2 volume fraction of 9%, CO2 is enriched to the volume fraction of 65.88% by the first-stage VPSA, and then purified to the volume fraction of 95.31% by the second-stage VPSA, achieving a total CO2 recovery rate of 92.20%. By recycling the exhaust gas from the adsorption phase of the second-stage VPSA into the first-stage feed, the total CO2 recovery rate increases to 95.20%. The CO2 purity (> 95%) meets stringent requirements for battery-grade lithium carbonate production via carbonation. This study can provide a theoretical foundation for CO2 separation and resource utilization from roasting kiln exhaust gas.关键词:lithium concentrate roasting kiln exhaust gas;CO2;VPSA;process simulation;13X zeolite43|0|0更新时间:2025-07-30
摘要:The application of pressure swing adsorption (PSA) to extract CO₂ from the exhaust gas of lithium ore roasting (natural gas) furnaces and employ it as a carbonization feedstock in the production of lithium carbonate from spodumene has garnered extensive interest. Considering the low CO2 concentration and elevated temperature characteristics of the exhaust gas, a two-stage vacuum pressure swing adsorption (VPSA) process was specifically designed, and the process was simulated using Aspen Adsorption software. The results show that using 13X zeolite as the adsorbent under feed gas (roasting kiln exhaust gas) temperature of 373.15 K, regeneration pressure of 5 kPa, and CO2 volume fraction of 9%, CO2 is enriched to the volume fraction of 65.88% by the first-stage VPSA, and then purified to the volume fraction of 95.31% by the second-stage VPSA, achieving a total CO2 recovery rate of 92.20%. By recycling the exhaust gas from the adsorption phase of the second-stage VPSA into the first-stage feed, the total CO2 recovery rate increases to 95.20%. The CO2 purity (> 95%) meets stringent requirements for battery-grade lithium carbonate production via carbonation. This study can provide a theoretical foundation for CO2 separation and resource utilization from roasting kiln exhaust gas.关键词:lithium concentrate roasting kiln exhaust gas;CO2;VPSA;process simulation;13X zeolite43|0|0更新时间:2025-07-30 -
 摘要:At present, China has the problem of insufficient capacity of conventional natural gas, and it needs to develop unconventional natural gas such as coalbed methane as a supplement. In the process of coal mining, a large amount of air will be mixed to form low-concentration coalbed methane (methane volume fraction is less than 30%), which leads to a series of problems such as resource waste. Therefore, improving the recovery and utilization rate of low-concentration coalbed methane has become an urgent problem to be solved. The adsorption and separation of CH4/N2 in low concentration coalbed methane was studied by using the combination of Grand canonical Monte Carlo and density functional theory. Cu-based metal organic framework (MOFs) (Cu-BTC, MOF-143, ATC-Cu and MOF-399) and ATC-M modified by different metals (Zn, Co and Mo) were selected as adsorption materials.The effects of different pore sizes and metal centers on the adsorption and separation performances of CH4/N2 by MOFs were studied. The results show that the pore size of MOFs has a significant impact on their adsorption capacity and CH4/N2 adsorption selectivity. The closer the pore size of MOFs is to the molecular kinetic diameter of the gas, the higher the CH4/N2 adsorption selectivity. Among the seven MOFs, ATC-Zn has a pore size (0.4995 nm) that is closer to the molecular kinetic diameters of CH4 and N2 (CH4: 0.380 nm, N2: 0.364 nm), achieving the best CH4/N2 separation performance. Metal center modification has no significant effect on the pore size of ATC-M. The charge of the modified metal center is the main factor affecting the CH4/N2 adsorption and separation performance. The larger the bader charge of the metal ion, the better the CH4/N2 adsorption and separation performance of MOFs. Among the seven MOFs, ATC-Zn has the best CH4/N2 adsorption and separation performance. At 298 K and 100 kPa, when n(CH4):n(N2) of low-concentration coalbed methane is 1:9, the CH4/N2 adsorption selectivity of ATC-Zn reaches as high as 6.46, providing a new research idea for the recovery and utilization of CH4 in low-concentration coalbed methane.关键词:Cu-based MOFs;CH4/N2;adsorption separation;low-concentration coalbed methane;pore size;charge93|0|0更新时间:2025-07-30
摘要:At present, China has the problem of insufficient capacity of conventional natural gas, and it needs to develop unconventional natural gas such as coalbed methane as a supplement. In the process of coal mining, a large amount of air will be mixed to form low-concentration coalbed methane (methane volume fraction is less than 30%), which leads to a series of problems such as resource waste. Therefore, improving the recovery and utilization rate of low-concentration coalbed methane has become an urgent problem to be solved. The adsorption and separation of CH4/N2 in low concentration coalbed methane was studied by using the combination of Grand canonical Monte Carlo and density functional theory. Cu-based metal organic framework (MOFs) (Cu-BTC, MOF-143, ATC-Cu and MOF-399) and ATC-M modified by different metals (Zn, Co and Mo) were selected as adsorption materials.The effects of different pore sizes and metal centers on the adsorption and separation performances of CH4/N2 by MOFs were studied. The results show that the pore size of MOFs has a significant impact on their adsorption capacity and CH4/N2 adsorption selectivity. The closer the pore size of MOFs is to the molecular kinetic diameter of the gas, the higher the CH4/N2 adsorption selectivity. Among the seven MOFs, ATC-Zn has a pore size (0.4995 nm) that is closer to the molecular kinetic diameters of CH4 and N2 (CH4: 0.380 nm, N2: 0.364 nm), achieving the best CH4/N2 separation performance. Metal center modification has no significant effect on the pore size of ATC-M. The charge of the modified metal center is the main factor affecting the CH4/N2 adsorption and separation performance. The larger the bader charge of the metal ion, the better the CH4/N2 adsorption and separation performance of MOFs. Among the seven MOFs, ATC-Zn has the best CH4/N2 adsorption and separation performance. At 298 K and 100 kPa, when n(CH4):n(N2) of low-concentration coalbed methane is 1:9, the CH4/N2 adsorption selectivity of ATC-Zn reaches as high as 6.46, providing a new research idea for the recovery and utilization of CH4 in low-concentration coalbed methane.关键词:Cu-based MOFs;CH4/N2;adsorption separation;low-concentration coalbed methane;pore size;charge93|0|0更新时间:2025-07-30 -
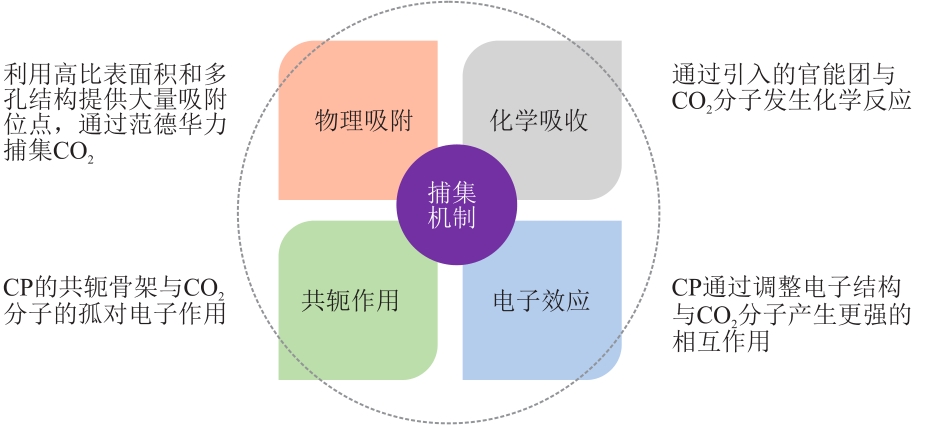 摘要:Conjugated polymers (CP) have been applied in the field of CO2 capture due to their unique conjugated framework, permanent nanoporous structure, tunable properties and large specific surface area. The mechanisms of CO2 capture by CP were reviewed, which mainly involve four types: physical adsorption, chemical absorption, conjugation effect and electronic effect. The key factors influencing CP synthesis were analyzed, including monomer structure, introduction of functional groups, choice of preparation solvents and coupling reaction methods, all of which directly affect the functional properties of CP materials. Additionally, recent research progress of CO2 capture by CP was summarized. To address challenges such as low regeneration capacity, high energy consumption and limited selectivity, strategies such as optimizing traditional coupling methods (e.g., Stille and Sonogashira) and introducing specific functional groups have been proposed to regulate the microporous structue of CP, thereby enhancing their CO2 capture performances.关键词:capture mechanisms;conjugated polymers;CO2 capture92|0|0更新时间:2025-07-30
摘要:Conjugated polymers (CP) have been applied in the field of CO2 capture due to their unique conjugated framework, permanent nanoporous structure, tunable properties and large specific surface area. The mechanisms of CO2 capture by CP were reviewed, which mainly involve four types: physical adsorption, chemical absorption, conjugation effect and electronic effect. The key factors influencing CP synthesis were analyzed, including monomer structure, introduction of functional groups, choice of preparation solvents and coupling reaction methods, all of which directly affect the functional properties of CP materials. Additionally, recent research progress of CO2 capture by CP was summarized. To address challenges such as low regeneration capacity, high energy consumption and limited selectivity, strategies such as optimizing traditional coupling methods (e.g., Stille and Sonogashira) and introducing specific functional groups have been proposed to regulate the microporous structue of CP, thereby enhancing their CO2 capture performances.关键词:capture mechanisms;conjugated polymers;CO2 capture92|0|0更新时间:2025-07-30 -
 摘要:The amine-based absorption method is a promising technology for CO2 capture, but the high regeneration energy consumption increases its costs. Developing solid acid catalysts with high catalytic activity and stability is a key approach to reducing the regeneration energy consumption of amine solutions. A series of bimetal-loaded γ-Al2O3 solid acid catalysts ZrM/γ-Al2O3 (M = Ni, Fe, Ce or Cu, mass fractions of Zr and M both are 10%) were prepared by loading two types of metals on the support γ-Al2O3 using the impregnation method. And the catalytic desorption performances and mechanisms of the catalysts in monoethanolamine rich solution with CO2 (MEA rich solution) were investigated. The results show that under the conditions of desorption temperature of 91 ℃, stirring rate of 500 r/min and CO2 loading of 0.5 mol/mol, ZrNi/γ-Al2O3 exhibits the most favorable catalytic desorption performance. Specifically, the peak CO2 desorption rate, CO2 total desorption amount and relative energy consumption (compared to the blank group without catalyst) catalyzed by ZrNi/γ-Al2O3 are 1.92 mmol/min, 46.53 mmol and 55.04%, respectively. After eight absorption-desorption cycles at 40 ℃ for absorption and 92 ℃ for desorption, ZrNi/γ-Al2O3 maintains good catalytic desorption performance with reducing the regeneration energy consumption of MEA rich solution by approximately 23% compared to the blank group. This indicates that ZrNi/γ-Al2O3 possesses good cyclic stability. The characterization results of N2 adsorption/desorption, NH3-TPD and Py-IR show that ZrM/γ-Al2O3 catalysts demonstrate suitable textural properties and acidity. The abundant Brønsted and Lewis acid sites play critical role in the catalytic desorption process of MEA rich solutions.关键词:CO2 capture;catalytic desorption;bimetallic supported γ-Al2O3;solid acid catalysts;desorption mechanism274|0|0更新时间:2025-07-30
摘要:The amine-based absorption method is a promising technology for CO2 capture, but the high regeneration energy consumption increases its costs. Developing solid acid catalysts with high catalytic activity and stability is a key approach to reducing the regeneration energy consumption of amine solutions. A series of bimetal-loaded γ-Al2O3 solid acid catalysts ZrM/γ-Al2O3 (M = Ni, Fe, Ce or Cu, mass fractions of Zr and M both are 10%) were prepared by loading two types of metals on the support γ-Al2O3 using the impregnation method. And the catalytic desorption performances and mechanisms of the catalysts in monoethanolamine rich solution with CO2 (MEA rich solution) were investigated. The results show that under the conditions of desorption temperature of 91 ℃, stirring rate of 500 r/min and CO2 loading of 0.5 mol/mol, ZrNi/γ-Al2O3 exhibits the most favorable catalytic desorption performance. Specifically, the peak CO2 desorption rate, CO2 total desorption amount and relative energy consumption (compared to the blank group without catalyst) catalyzed by ZrNi/γ-Al2O3 are 1.92 mmol/min, 46.53 mmol and 55.04%, respectively. After eight absorption-desorption cycles at 40 ℃ for absorption and 92 ℃ for desorption, ZrNi/γ-Al2O3 maintains good catalytic desorption performance with reducing the regeneration energy consumption of MEA rich solution by approximately 23% compared to the blank group. This indicates that ZrNi/γ-Al2O3 possesses good cyclic stability. The characterization results of N2 adsorption/desorption, NH3-TPD and Py-IR show that ZrM/γ-Al2O3 catalysts demonstrate suitable textural properties and acidity. The abundant Brønsted and Lewis acid sites play critical role in the catalytic desorption process of MEA rich solutions.关键词:CO2 capture;catalytic desorption;bimetallic supported γ-Al2O3;solid acid catalysts;desorption mechanism274|0|0更新时间:2025-07-30 -
 摘要:To achieve the goal of “carbon neutrality”, CO2 direct air capture (DAC) technology is receiving increasing attention. The adsorption-based direct air capture system was designed and constructed, utilizing a primary amine-grafted ion exchange resin as the CO2 adsorbent. Under the same adsorption bed conditions, a comparative experimental study was conducted on two typical thermal cycles: Temperature vacuum swing adsorption (TVSA) and steam-assisted temperature vacuum swing adsorption (S-TVSA). The experimental results show that, at an ambient temperature of 20~32 ℃, both cycle modes can achieve more than 50% CO2 capture ratio and obtain CO2 product gas with purity (mole fraction) higher than 90%. Under the desorption condition of 100~120 ℃, continuous steam fed at a flow rate of 1 m3/h, the product gas productivity of the S-TVSA cycle can reach 0.33 kg/(kg·d), which is much higher than that of TVSA cycle (0.15 kg/(kg·d)) under regeneration temperature of 70 ℃ and vacuum pressure of 3 kPa. The regeneration energy consumption of S-TVSA cycle is much lower than that of TVSA cycle. For 1 t of CO2 produced, the regeneration energy consumption of S-TVSA cycle is 3.56 GJ, which is only 1/6 of that of TVSA cycle. The research results show that when the selected solid amine adsorbent is used, under similar environmental conditions and within the same cycle period, the heat and mass transfer efficiency of the S-TVSA cycle is higher, the desorption process is relatively thorough, and the adsorption potential of the adsorbent is fully utilized, making it more advantageous compared to the TVSA cycle.关键词:CO2 direct air capture;adsorption;desorption;cycle;energy consumption192|0|0更新时间:2025-07-30
摘要:To achieve the goal of “carbon neutrality”, CO2 direct air capture (DAC) technology is receiving increasing attention. The adsorption-based direct air capture system was designed and constructed, utilizing a primary amine-grafted ion exchange resin as the CO2 adsorbent. Under the same adsorption bed conditions, a comparative experimental study was conducted on two typical thermal cycles: Temperature vacuum swing adsorption (TVSA) and steam-assisted temperature vacuum swing adsorption (S-TVSA). The experimental results show that, at an ambient temperature of 20~32 ℃, both cycle modes can achieve more than 50% CO2 capture ratio and obtain CO2 product gas with purity (mole fraction) higher than 90%. Under the desorption condition of 100~120 ℃, continuous steam fed at a flow rate of 1 m3/h, the product gas productivity of the S-TVSA cycle can reach 0.33 kg/(kg·d), which is much higher than that of TVSA cycle (0.15 kg/(kg·d)) under regeneration temperature of 70 ℃ and vacuum pressure of 3 kPa. The regeneration energy consumption of S-TVSA cycle is much lower than that of TVSA cycle. For 1 t of CO2 produced, the regeneration energy consumption of S-TVSA cycle is 3.56 GJ, which is only 1/6 of that of TVSA cycle. The research results show that when the selected solid amine adsorbent is used, under similar environmental conditions and within the same cycle period, the heat and mass transfer efficiency of the S-TVSA cycle is higher, the desorption process is relatively thorough, and the adsorption potential of the adsorbent is fully utilized, making it more advantageous compared to the TVSA cycle.关键词:CO2 direct air capture;adsorption;desorption;cycle;energy consumption192|0|0更新时间:2025-07-30 -
 摘要:As a clean secondary energy source, hydrogen energy can provide a reference for solving China’s energy problems. Photolysis of water to hydrogen has attracted much attention because it can directly use sunlight to split water to H2 and O2. Finding suitable photocatalyst is the key to photolysis of water to produce hydrogen. CdS has good photocatalytic activity, however, there are still some problems, such as the need for further optimization of the band structure, photocorrosion and high photogenerated carrier recombination rate. The mechanism of CdS photocatalyst for hydrogen production by photolysis of water was summarized, and a reference solution to the existing problems of CdS photocatalyst was gived. The reasonable band structure of the catalyst can be obtained by element doping, and the photocorrosion resistance can be improved by designing the protective layer and removing O2, and the photogenerated carrier migration efficiency can be increased by constructing the built-in electric field, designing the active site and morphology control technology.关键词:CdS photocatalyst;photolysis of water to produce hydrogen;band structure;photocorrosion;photogenerated carrier273|0|0更新时间:2025-07-30
摘要:As a clean secondary energy source, hydrogen energy can provide a reference for solving China’s energy problems. Photolysis of water to hydrogen has attracted much attention because it can directly use sunlight to split water to H2 and O2. Finding suitable photocatalyst is the key to photolysis of water to produce hydrogen. CdS has good photocatalytic activity, however, there are still some problems, such as the need for further optimization of the band structure, photocorrosion and high photogenerated carrier recombination rate. The mechanism of CdS photocatalyst for hydrogen production by photolysis of water was summarized, and a reference solution to the existing problems of CdS photocatalyst was gived. The reasonable band structure of the catalyst can be obtained by element doping, and the photocorrosion resistance can be improved by designing the protective layer and removing O2, and the photogenerated carrier migration efficiency can be increased by constructing the built-in electric field, designing the active site and morphology control technology.关键词:CdS photocatalyst;photolysis of water to produce hydrogen;band structure;photocorrosion;photogenerated carrier273|0|0更新时间:2025-07-30 -
 摘要:With the deterioration of energy problems, hydrogen has become one of the best new energy due to its advantages of green environmental protection, abundant resources and high energy density per unit mass. Hydrogen storage and transportation is the key to hydrogen energy research popularization, among which solid hydrogen storage materials have the advantages of large hydrogen storage capacity, high hydrogen storage density and good safety performance, making them the most promising hydrogen storage materials. The hydrogen storage properties of various solid hydrogen storage materials (carbon-based hydrogen storage materials, organic porous hydrogen storage materials, metal-based hydrogen storage materials and coordination hydride hydrogen storage materials) and current research status of solid hydrogen storage materials at home and abroad were reviewed. The future development direction of various solid hydrogen storage materials was prospected to further improve the hydrogen storage performance of solid hydrogen storage materials. The future research directions of solid hydrogen storage materials can focus on synthesizing multi-functional hydrogen storage materials, using advanced characterization methods to deeply analyze their properties and hydrogen storage mechanisms and improving industry standards and safety evaluation system to promote industry development.关键词:hydrogen energy;solid hydrogen storage materials;hydrogen storage performance1085|0|0更新时间:2025-07-30
摘要:With the deterioration of energy problems, hydrogen has become one of the best new energy due to its advantages of green environmental protection, abundant resources and high energy density per unit mass. Hydrogen storage and transportation is the key to hydrogen energy research popularization, among which solid hydrogen storage materials have the advantages of large hydrogen storage capacity, high hydrogen storage density and good safety performance, making them the most promising hydrogen storage materials. The hydrogen storage properties of various solid hydrogen storage materials (carbon-based hydrogen storage materials, organic porous hydrogen storage materials, metal-based hydrogen storage materials and coordination hydride hydrogen storage materials) and current research status of solid hydrogen storage materials at home and abroad were reviewed. The future development direction of various solid hydrogen storage materials was prospected to further improve the hydrogen storage performance of solid hydrogen storage materials. The future research directions of solid hydrogen storage materials can focus on synthesizing multi-functional hydrogen storage materials, using advanced characterization methods to deeply analyze their properties and hydrogen storage mechanisms and improving industry standards and safety evaluation system to promote industry development.关键词:hydrogen energy;solid hydrogen storage materials;hydrogen storage performance1085|0|0更新时间:2025-07-30 -
 摘要:Utilizing the existing natural gas pipelines for hydrogen blending is an effective method for achieving large-scale, long-distance, and low-cost storage and transportation of hydrogen. Developing efficient and convenient hydrogen blending device and improving the blending uniformity of hydrogen and natural gas can improve hydrogen transportation efficiency and ensure long-distance pipeline transportation and downstream gas safety. Using a Venturi tube as the hydrogen blending device, methane was used instead of natural gas to mix methane and hydrogen. By numerical simulation method, the influence of different blending structures and operating conditions on the flow process and blending uniformity of methane and hydrogen was studied. The results show that adding a Venturi tube after T-shaped pipeline can effectively improve the blending uniformity. Under the simulated operating conditions, the best blending effect is achieved when the tube-throat ratio (diameter ratio of throat section to straight section of the Venturi tube) is 1/3. When the hydrogen blending ratio (mass fraction ratio of hydrogen to methane) is 15%, the blending effect is the best, and the blending uniformity increases with the increase of the hydrogen blending ratio. Compared with static mixing devices, even with low methane flow rates, the pipeline with added a Venturi tube can still maintain great mixing uniformity. The lower the operating pressure, the smaller the fluctuation of blending uniformity, and the more stable the blending process.关键词:hydrogen-blending natural gas;Venturi tube;blending uniformity;hydrogen blending ratio;fluid flow law228|0|0更新时间:2025-07-30
摘要:Utilizing the existing natural gas pipelines for hydrogen blending is an effective method for achieving large-scale, long-distance, and low-cost storage and transportation of hydrogen. Developing efficient and convenient hydrogen blending device and improving the blending uniformity of hydrogen and natural gas can improve hydrogen transportation efficiency and ensure long-distance pipeline transportation and downstream gas safety. Using a Venturi tube as the hydrogen blending device, methane was used instead of natural gas to mix methane and hydrogen. By numerical simulation method, the influence of different blending structures and operating conditions on the flow process and blending uniformity of methane and hydrogen was studied. The results show that adding a Venturi tube after T-shaped pipeline can effectively improve the blending uniformity. Under the simulated operating conditions, the best blending effect is achieved when the tube-throat ratio (diameter ratio of throat section to straight section of the Venturi tube) is 1/3. When the hydrogen blending ratio (mass fraction ratio of hydrogen to methane) is 15%, the blending effect is the best, and the blending uniformity increases with the increase of the hydrogen blending ratio. Compared with static mixing devices, even with low methane flow rates, the pipeline with added a Venturi tube can still maintain great mixing uniformity. The lower the operating pressure, the smaller the fluctuation of blending uniformity, and the more stable the blending process.关键词:hydrogen-blending natural gas;Venturi tube;blending uniformity;hydrogen blending ratio;fluid flow law228|0|0更新时间:2025-07-30
0

