最新刊期
卷 50 , 期 10 , 2025
-
 摘要:Carbon nanotubes are tubular nanomaterials formed by the coiling of layered graphite, which are widely used in many fields because of high mechanical strength, excellent conductivity, good biocompatibility, stable chemical property and large specific surface area. Chemical vapor deposition (CVD) method is one of the key technologies for the synthesis of carbon nanotubes, and has become the mainstream technology for large-scale synthesis of high-performance carbon nanotubes due to its controllability and high yield. Ni-based catalysts are widely used for methane cracking due to their high catalytic activity and product selectivity. The growth mechanisms of multi-walled carbon nanotubes (MWCNTs) catalyzed by Ni-based catalysts for methane cracking and the regulation of MWCNTs structures by different types of Ni-based catalysts were summarized. Furthermore, the controllable growth of MWCNTs catalyzed by Ni-based catalysts for methane cracking was prospected, in order to provide guidance for the relevant research on methane cracking to prepare MWCNTs.关键词:chemical vapor deposition method;Ni-based catalysts;methane cracking;multi-walled carbon nanotubes146|0|0更新时间:2025-10-22
摘要:Carbon nanotubes are tubular nanomaterials formed by the coiling of layered graphite, which are widely used in many fields because of high mechanical strength, excellent conductivity, good biocompatibility, stable chemical property and large specific surface area. Chemical vapor deposition (CVD) method is one of the key technologies for the synthesis of carbon nanotubes, and has become the mainstream technology for large-scale synthesis of high-performance carbon nanotubes due to its controllability and high yield. Ni-based catalysts are widely used for methane cracking due to their high catalytic activity and product selectivity. The growth mechanisms of multi-walled carbon nanotubes (MWCNTs) catalyzed by Ni-based catalysts for methane cracking and the regulation of MWCNTs structures by different types of Ni-based catalysts were summarized. Furthermore, the controllable growth of MWCNTs catalyzed by Ni-based catalysts for methane cracking was prospected, in order to provide guidance for the relevant research on methane cracking to prepare MWCNTs.关键词:chemical vapor deposition method;Ni-based catalysts;methane cracking;multi-walled carbon nanotubes146|0|0更新时间:2025-10-22 -
 摘要:Carbon dioxide (CO2) and methane (CH4) are the main greenhouse gases that cause global warming. Dry reforming (DRM) of CO2 and CH4 to syngas is one of the feasible solutions to greenhouse gas problem, but the catalyst is easily deactivated due to carbon deposition and metal sintering. Catalysts 6NiO/AC, 6NiO/HZSM-5 and 6NiO/SA with nickel mass fraction of 6% were prepared by ultrasonic impregnation method using activated carbon (AC), HZMS-5 and aluminum slag as carriers. The catalytic performance of these catalysts for DRM reaction was studied in a fixed bed reactor, and the catalysts were characterized by XRD, N2 adsorption/desorption, TG and SEM. The results show that the catalytic activity of 6NiO/SA is the lowest among the three catalysts. 6NiO/AC has the largest specific surface area and smaller metal grains, showing better catalytic activity than 6NiO/HZSM-5 and 6NiO/SA. Under the conditions of n(CO2)/n(CH4) of 1, the temperature of 900 ℃ and the reaction time of 60 min, the CH4 conversion rate and CO2 conversion rate are 91.4% and 93.4%, respectively. The stability of 6NiO/AC was investigated under the conditions of n(CO2)/n(CH4) of 1, temperature of 800 ℃ and reaction time of 240 min. It is found that the CH4 conversion rate is always maintained at about 70%. After the reaction, the carbon deposition type of 6NiO/AC is mainly filamentous carbon deposition, which ensures the active site of the catalyst. In addition, the introduction of excessive CO2 in the reaction system can make the reaction go forward, promote the carbon deposition gasification reaction and inhibit the carbon formation.关键词:carbon dioxide-methane reforming;nickel-based catalysts;activated carbon;supports161|0|0更新时间:2025-10-22
摘要:Carbon dioxide (CO2) and methane (CH4) are the main greenhouse gases that cause global warming. Dry reforming (DRM) of CO2 and CH4 to syngas is one of the feasible solutions to greenhouse gas problem, but the catalyst is easily deactivated due to carbon deposition and metal sintering. Catalysts 6NiO/AC, 6NiO/HZSM-5 and 6NiO/SA with nickel mass fraction of 6% were prepared by ultrasonic impregnation method using activated carbon (AC), HZMS-5 and aluminum slag as carriers. The catalytic performance of these catalysts for DRM reaction was studied in a fixed bed reactor, and the catalysts were characterized by XRD, N2 adsorption/desorption, TG and SEM. The results show that the catalytic activity of 6NiO/SA is the lowest among the three catalysts. 6NiO/AC has the largest specific surface area and smaller metal grains, showing better catalytic activity than 6NiO/HZSM-5 and 6NiO/SA. Under the conditions of n(CO2)/n(CH4) of 1, the temperature of 900 ℃ and the reaction time of 60 min, the CH4 conversion rate and CO2 conversion rate are 91.4% and 93.4%, respectively. The stability of 6NiO/AC was investigated under the conditions of n(CO2)/n(CH4) of 1, temperature of 800 ℃ and reaction time of 240 min. It is found that the CH4 conversion rate is always maintained at about 70%. After the reaction, the carbon deposition type of 6NiO/AC is mainly filamentous carbon deposition, which ensures the active site of the catalyst. In addition, the introduction of excessive CO2 in the reaction system can make the reaction go forward, promote the carbon deposition gasification reaction and inhibit the carbon formation.关键词:carbon dioxide-methane reforming;nickel-based catalysts;activated carbon;supports161|0|0更新时间:2025-10-22 -
 摘要:The dry reforming of methane (DRM) reaction efficiently converts two major greenhouse gases, CO2 and CH4, into H2 and CO, thereby achieving resource utilization and holding significant research value. Ni-based catalysts have garnered attention due to their low cost and high activity, but their deactivation caused by coking remains a pressing issue. Three-dimensional mesoporous Ni-ZrO2 catalysts were prepared by colloidal silica-assisted solution combustion method and applied to low-temperature DRM reaction. Characterizations including N2 adsorption/desorption, XRD, H2-TPR, TEM, and TG-DTA were conducted to systematically investigate the structures and the effects of Ni contents (mass fraction, the same below) and reaction temperatures on catalytic performance of catalysts. Comparisons were made with catalysts prepared via ordinary impregnation method and traditional solution combustion method. The results indicate that the catalysts prepared by colloidal silica-assisted solution combustion method exhibit larger specific surface areas, stable three-dimensional mesoporous structures, and have strong metal-support interactions. Regarding catalytic performance, Ni contents significantly affect both anti-coking performances and catalytic activities. As the Ni contents increase, the tendency of catalysts for coking becomes more pronounced. After a 20 h low-temperature DRM reaction at the temperature of 600 ℃ and space velocity of 135000 mL/(g·h), the three-dimensional mesoporous 1%Ni-ZrO2-CSC catalyst maintains CH4 and CO2 conversion rates of 31% and 38%, respectively. The n(H2)/n(CO) stabilizes at 0.45, and no coking is observed, demonstrating 1%Ni-ZrO2-CSC’s excellent resistance to coking.关键词:dry reforming of methane;Ni-ZrO2;catalysts;colloidal silica-assisted solution combustion method;three-dimensional mesoporous141|0|0更新时间:2025-10-22
摘要:The dry reforming of methane (DRM) reaction efficiently converts two major greenhouse gases, CO2 and CH4, into H2 and CO, thereby achieving resource utilization and holding significant research value. Ni-based catalysts have garnered attention due to their low cost and high activity, but their deactivation caused by coking remains a pressing issue. Three-dimensional mesoporous Ni-ZrO2 catalysts were prepared by colloidal silica-assisted solution combustion method and applied to low-temperature DRM reaction. Characterizations including N2 adsorption/desorption, XRD, H2-TPR, TEM, and TG-DTA were conducted to systematically investigate the structures and the effects of Ni contents (mass fraction, the same below) and reaction temperatures on catalytic performance of catalysts. Comparisons were made with catalysts prepared via ordinary impregnation method and traditional solution combustion method. The results indicate that the catalysts prepared by colloidal silica-assisted solution combustion method exhibit larger specific surface areas, stable three-dimensional mesoporous structures, and have strong metal-support interactions. Regarding catalytic performance, Ni contents significantly affect both anti-coking performances and catalytic activities. As the Ni contents increase, the tendency of catalysts for coking becomes more pronounced. After a 20 h low-temperature DRM reaction at the temperature of 600 ℃ and space velocity of 135000 mL/(g·h), the three-dimensional mesoporous 1%Ni-ZrO2-CSC catalyst maintains CH4 and CO2 conversion rates of 31% and 38%, respectively. The n(H2)/n(CO) stabilizes at 0.45, and no coking is observed, demonstrating 1%Ni-ZrO2-CSC’s excellent resistance to coking.关键词:dry reforming of methane;Ni-ZrO2;catalysts;colloidal silica-assisted solution combustion method;three-dimensional mesoporous141|0|0更新时间:2025-10-22 -
 摘要:As an important chemical raw material, phenylethanol can be prepared by catalytic hydrogenation of acetophenone. However, the currently used catalysts for hydrogenation of acetophenone to phenylethanol have problems such as low product selectivities, poor stabilities and high costs. Cu-Mn catalysts (Cu-MnOx-MT and Cu-MnOx-CP) were prepared by melting method and co precipitation method, respectively. The structures of catalysts were characterized by XRD, H2-TPR, etc. The catalytic performances of catalysts were studied and the reaction conditions were optimized. The results show that compared with Cu-MnOx-CP, Cu-MnOx-MT has more abundant oxygen vacancy active sites, which are conducive to enhancing the migration of surface oxygen, increasing the adsorption energy of acetophenone on the catalyst surface, reducing the reaction energy barrier, promoting the activation of acetophenone, and thus improving the acetophenone conversion rate. Under the optimal conditions of reaction temperature of 100 ℃, reaction pressure of 2.5 MPa, liquid space velocity of 1.8 h-1 and V(H2):V(acetophenone) of 15:1, Cu-MnOx-MT exhibits better catalytic performance with the acetophenone conversion rate of 99.75% and phenylethanol selectivity of 99.83% after reacting for 8 h. In addition, after continuously reacting under optimal reaction conditions for 40 h, the acetophenone conversion rate of Cu-MnOx-MT remains above 99%.关键词:acetophenone;phenylethanol;Cu-Mn catalysts;melting method161|0|0更新时间:2025-10-22
摘要:As an important chemical raw material, phenylethanol can be prepared by catalytic hydrogenation of acetophenone. However, the currently used catalysts for hydrogenation of acetophenone to phenylethanol have problems such as low product selectivities, poor stabilities and high costs. Cu-Mn catalysts (Cu-MnOx-MT and Cu-MnOx-CP) were prepared by melting method and co precipitation method, respectively. The structures of catalysts were characterized by XRD, H2-TPR, etc. The catalytic performances of catalysts were studied and the reaction conditions were optimized. The results show that compared with Cu-MnOx-CP, Cu-MnOx-MT has more abundant oxygen vacancy active sites, which are conducive to enhancing the migration of surface oxygen, increasing the adsorption energy of acetophenone on the catalyst surface, reducing the reaction energy barrier, promoting the activation of acetophenone, and thus improving the acetophenone conversion rate. Under the optimal conditions of reaction temperature of 100 ℃, reaction pressure of 2.5 MPa, liquid space velocity of 1.8 h-1 and V(H2):V(acetophenone) of 15:1, Cu-MnOx-MT exhibits better catalytic performance with the acetophenone conversion rate of 99.75% and phenylethanol selectivity of 99.83% after reacting for 8 h. In addition, after continuously reacting under optimal reaction conditions for 40 h, the acetophenone conversion rate of Cu-MnOx-MT remains above 99%.关键词:acetophenone;phenylethanol;Cu-Mn catalysts;melting method161|0|0更新时间:2025-10-22 -
 摘要:The direct synthesis of dimethyl carbonate (DMC) with carbon dioxide (CO2) and methanol (CH3OH) is currently one of the most environmentally advantageous methods for DMC production. Ce based catalysts are commonly used in direct synthesis of DMC with CO2 and CH3OH, but CeO2 suffers from issues such as insufficient thermal stability and limited active sites. Using neodymium nitrate hexahydrate (NdN3O9·6H2O) as the promoter, a series of Nd-doped Ce-based catalysts were prepared by co-precipitation method. The structures of catalysts were characterized by ICP-OES, XRD, HR-TEM, and XPS, etc., and their catalytic performances were investigated. The results show that the appropriate Nd doping amount (mass fraction, the same below) can positively optimize the catalyst’s specific surface area, oxygen vacancy density and the number of acid-base sites. Among them, the catalyst with Nd doping amount of 5% (5% Nd2O3-CeO2) has the highest oxygen vacancy density, more medium-strong acid sites, and the most weak base sites. Under the reaction conditions of CH3OH dosage of 23.74 g, 2-cyanopyridine dosage of 36.44 g, 5% Nd2O3-CeO2 dosage of 200 mg, temperature of 140 °C and pressure of 1.2 MPa, 5% the CH3OH conversion rate is 27.35%, DMC selectivity is 77.72% and DMC yield is 392.90 mmol/g catalyzed by 5% Nd2O3-CeO2 after reacting for 2 h. After four cycles under the conditions of temperature of 140 ℃, pressure of 1.2 MPa, CH3OH dosage of 23.74 g, 5% Nd2O3-CeO2 dosage of 200 mg and reaction time of 2 h, the catalytic performance of 5% Nd2O3-CeO2 remains superior to that of CeO2.关键词:dimethyl carbonate;direct synthesis;Nd doping;Ce-based catalysts;carbon dioxide;methanol91|0|0更新时间:2025-10-22
摘要:The direct synthesis of dimethyl carbonate (DMC) with carbon dioxide (CO2) and methanol (CH3OH) is currently one of the most environmentally advantageous methods for DMC production. Ce based catalysts are commonly used in direct synthesis of DMC with CO2 and CH3OH, but CeO2 suffers from issues such as insufficient thermal stability and limited active sites. Using neodymium nitrate hexahydrate (NdN3O9·6H2O) as the promoter, a series of Nd-doped Ce-based catalysts were prepared by co-precipitation method. The structures of catalysts were characterized by ICP-OES, XRD, HR-TEM, and XPS, etc., and their catalytic performances were investigated. The results show that the appropriate Nd doping amount (mass fraction, the same below) can positively optimize the catalyst’s specific surface area, oxygen vacancy density and the number of acid-base sites. Among them, the catalyst with Nd doping amount of 5% (5% Nd2O3-CeO2) has the highest oxygen vacancy density, more medium-strong acid sites, and the most weak base sites. Under the reaction conditions of CH3OH dosage of 23.74 g, 2-cyanopyridine dosage of 36.44 g, 5% Nd2O3-CeO2 dosage of 200 mg, temperature of 140 °C and pressure of 1.2 MPa, 5% the CH3OH conversion rate is 27.35%, DMC selectivity is 77.72% and DMC yield is 392.90 mmol/g catalyzed by 5% Nd2O3-CeO2 after reacting for 2 h. After four cycles under the conditions of temperature of 140 ℃, pressure of 1.2 MPa, CH3OH dosage of 23.74 g, 5% Nd2O3-CeO2 dosage of 200 mg and reaction time of 2 h, the catalytic performance of 5% Nd2O3-CeO2 remains superior to that of CeO2.关键词:dimethyl carbonate;direct synthesis;Nd doping;Ce-based catalysts;carbon dioxide;methanol91|0|0更新时间:2025-10-22 -
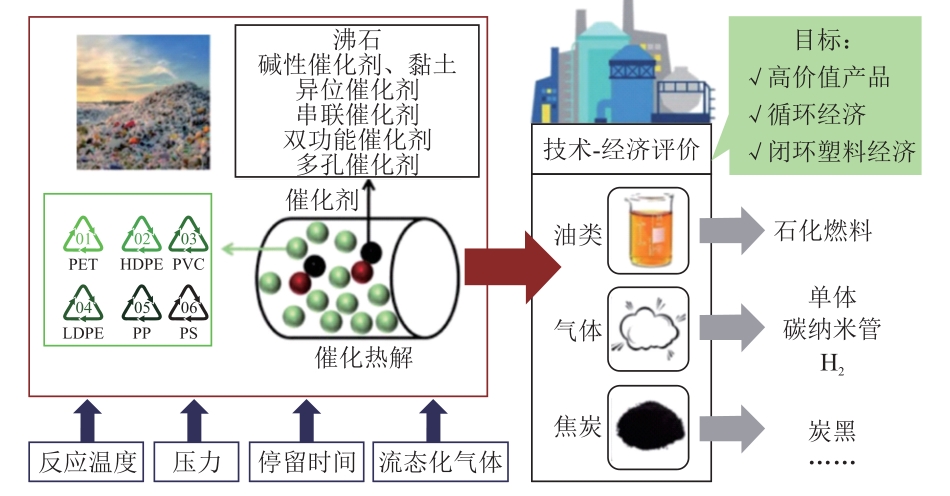 摘要:The resource utilization of waste plastics is an effective way to solve the environmental pollution problems caused by plastic waste, achieve the sustainable use of petrochemical resources and promote the development of “carbon peaking and carbon neutrality” policy. A review was conducted based on the most mainstream and typical catalytic pyrolysis waste plastics for resource utilization technology, and the technical route and reaction law were summarized. The catalytic pyrolysis optimization methods and challenges were deeply analyzed from the two perspectives of reactors (fixed bed reactor, fluidized bed reactor, conical jet bed reactor and free fall reactor) and catalysts (zeolite catalyst, fluidized catalytic cracking catalyst and activated carbon catalyst) used in catalytic pyrolysis. It is pointed out that accelerating the commercialization of catalytic pyrolysis waste plastic, constructing a complete industrial route for catalytic pyrolysis technology, measuring the feasibility of the carbon reduction path of catalytic pyrolysis technology, truly realizing the resource utilization of waste plastics, and helping China achieve the “carbon peaking and carbon neutrality” goal are the mainstream research directions in the future.关键词:catalytic pyrolysis;waste plastics;resource utilization;reactor;catalyst309|0|0更新时间:2025-10-22
摘要:The resource utilization of waste plastics is an effective way to solve the environmental pollution problems caused by plastic waste, achieve the sustainable use of petrochemical resources and promote the development of “carbon peaking and carbon neutrality” policy. A review was conducted based on the most mainstream and typical catalytic pyrolysis waste plastics for resource utilization technology, and the technical route and reaction law were summarized. The catalytic pyrolysis optimization methods and challenges were deeply analyzed from the two perspectives of reactors (fixed bed reactor, fluidized bed reactor, conical jet bed reactor and free fall reactor) and catalysts (zeolite catalyst, fluidized catalytic cracking catalyst and activated carbon catalyst) used in catalytic pyrolysis. It is pointed out that accelerating the commercialization of catalytic pyrolysis waste plastic, constructing a complete industrial route for catalytic pyrolysis technology, measuring the feasibility of the carbon reduction path of catalytic pyrolysis technology, truly realizing the resource utilization of waste plastics, and helping China achieve the “carbon peaking and carbon neutrality” goal are the mainstream research directions in the future.关键词:catalytic pyrolysis;waste plastics;resource utilization;reactor;catalyst309|0|0更新时间:2025-10-22 -
 摘要:At present, the world is focusing on low-carbon and sustainable energy. Biomass liquefaction, as a crucial pathway for renewable energy conversion, has attracted extensive attention. When traditional homogeneous catalysts are used in biomass liquefaction, it is difficult to separate and purify the bio-oil due to the co-phase with the bio-oil. Moreover, their strong acid-base properties are likely to cause serious environmental pollution problems. In order to solve the above problems, zeolites with great hydrothermal stability, high specific surface area and unique pore structure were selected as catalysts. The NaZSM-5 zeolite was modified through ion exchange method to prepare HZSM-5 zeolite. Characterization techniques including EA, XRD, FTIR and SEM were employed to analyze structural and morphological changes of zeolites before and after modification, using commercial HZSM-5 zeolite as reference. Employing HZSM-5 zeolite as catalyst, single-factor experiments were conducted to investigate the effects of various experimental conditions on liquefaction yield in rice straw liquefaction. The results show that the maximum liquefaction yield is 67.8% under optimal conditions: reaction temperature of 180 ℃, liquid-to-solid ratio (mass ratio of liquefaction agent to rice straw) of 5:1, reaction duration of 105 min, and catalyst dosage of 10% (mass percentage relative to liquefaction agent). Comparative analysis of physicochemical properties between bio-oil and crude oil reveales significant differences. The bio-oil exhibits distinctive characteristics including high viscosity, acidic nature, elevated hydroxyl value and carbon residue rate, demonstrating promising application prospects in chemical production fields.关键词:HZSM-5 zeolite;rice straw;atmospheric liquefaction;catalyst;bio-oil98|0|0更新时间:2025-10-22
摘要:At present, the world is focusing on low-carbon and sustainable energy. Biomass liquefaction, as a crucial pathway for renewable energy conversion, has attracted extensive attention. When traditional homogeneous catalysts are used in biomass liquefaction, it is difficult to separate and purify the bio-oil due to the co-phase with the bio-oil. Moreover, their strong acid-base properties are likely to cause serious environmental pollution problems. In order to solve the above problems, zeolites with great hydrothermal stability, high specific surface area and unique pore structure were selected as catalysts. The NaZSM-5 zeolite was modified through ion exchange method to prepare HZSM-5 zeolite. Characterization techniques including EA, XRD, FTIR and SEM were employed to analyze structural and morphological changes of zeolites before and after modification, using commercial HZSM-5 zeolite as reference. Employing HZSM-5 zeolite as catalyst, single-factor experiments were conducted to investigate the effects of various experimental conditions on liquefaction yield in rice straw liquefaction. The results show that the maximum liquefaction yield is 67.8% under optimal conditions: reaction temperature of 180 ℃, liquid-to-solid ratio (mass ratio of liquefaction agent to rice straw) of 5:1, reaction duration of 105 min, and catalyst dosage of 10% (mass percentage relative to liquefaction agent). Comparative analysis of physicochemical properties between bio-oil and crude oil reveales significant differences. The bio-oil exhibits distinctive characteristics including high viscosity, acidic nature, elevated hydroxyl value and carbon residue rate, demonstrating promising application prospects in chemical production fields.关键词:HZSM-5 zeolite;rice straw;atmospheric liquefaction;catalyst;bio-oil98|0|0更新时间:2025-10-22 -
 摘要:Hydrogen energy is the most widely applied secondary energy in renewable electricity consumption, but it faces challenges in “production, storage, transportation and utilization”. In the current and mid- to long-term carbon emission landscape, low-concentration CO2 (volume fraction < 20%) accounts for more than 50% of total emissions, making it a key area for carbon reduction governance. Focusing on these issues, the synergistic integration of industrial low-concentration CO2 with renewable electricity-driven water electrolysis for hydrogen production was proposed, establishing a catalytic reaction system for green methanol synthesis. This pathway can not only utilize methanol as a green and safe liquid hydrogen carrier, but also deliver significant carbon reduction benefits. To address the severe temporal mismatch between renewable power volatility and chemical production stability, a multi-mode flexible methanol production technology is innovatively developed: Adopting a modular architecture to achieve differentiated steady-state operation of different processes, constructing a multi-timescale cooperative optimization model that integrates second-level power fluctuations with hour-level chemical process dynamic responses, developing a multi-objective constrained optimization algorithm to balance carbon reduction benefits and economic performance. Taking a typical wind farm in Northeast China as a case study, system simulation and cost analysis verify the feasibility of the proposed technology under fluctuating power input conditions, and the levelized methanol production cost is quantified as 3832.26 CNY/t. The research can provide a technically feasible and economically rational solution for efficient conversion of industrial carbon sources.关键词:renewable electricity;hydrogen;carbon dioxide;multi-mode;methanol12|0|0更新时间:2025-10-22
摘要:Hydrogen energy is the most widely applied secondary energy in renewable electricity consumption, but it faces challenges in “production, storage, transportation and utilization”. In the current and mid- to long-term carbon emission landscape, low-concentration CO2 (volume fraction < 20%) accounts for more than 50% of total emissions, making it a key area for carbon reduction governance. Focusing on these issues, the synergistic integration of industrial low-concentration CO2 with renewable electricity-driven water electrolysis for hydrogen production was proposed, establishing a catalytic reaction system for green methanol synthesis. This pathway can not only utilize methanol as a green and safe liquid hydrogen carrier, but also deliver significant carbon reduction benefits. To address the severe temporal mismatch between renewable power volatility and chemical production stability, a multi-mode flexible methanol production technology is innovatively developed: Adopting a modular architecture to achieve differentiated steady-state operation of different processes, constructing a multi-timescale cooperative optimization model that integrates second-level power fluctuations with hour-level chemical process dynamic responses, developing a multi-objective constrained optimization algorithm to balance carbon reduction benefits and economic performance. Taking a typical wind farm in Northeast China as a case study, system simulation and cost analysis verify the feasibility of the proposed technology under fluctuating power input conditions, and the levelized methanol production cost is quantified as 3832.26 CNY/t. The research can provide a technically feasible and economically rational solution for efficient conversion of industrial carbon sources.关键词:renewable electricity;hydrogen;carbon dioxide;multi-mode;methanol12|0|0更新时间:2025-10-22 -
 摘要:The production of green hydrogen using renewable energy to synthesize green methanol can solve the problem of renewable energy consumption and is also one of the important ways to reduce carbon dioxide emissions. In order to design a green methanol project and assess its technical and economic feasibility, a case study of a green methanol project in Northeast China was conducted. The technical route and process flow of biomass gasification coupled with renewable energy hydrogen production for green methanol synthesis were evaluated. Considering the volatility of renewable energy generation, time-period scheduling control was applied. With the goal of optimizing the project’s economic performance, the real-time power balance method was simulated and matched, resulting in a hydrogen production capacity of 52000 m3/h for a 500 MW wind power installation. Additionally, the effective hydrogen storage capacity is 2210000 m3, and the green methanol production capacity is 160000 t/a. Under the given boundary conditions, internal rate of return (IRR), payback period, total return on investment,and net profit margin on equity were selected as the economic indicators. With a project financial IRR (after tax) of 6%, the green methanol price is calculated to be 5324.06 CNY/t. Additionally, the payback period (after tax) is 12.25 a, the financial IRR on equity is 11.91%, the total return on investment is 4.19%, and the net profit margin on equity is 12.59%. The project is economically viable. The proposed project plan is feasible, meets relevant standards and engineering requirements, and can provide a reference for future green methanol project design.关键词:renewable energy;green methanol;biomass gasification;hydrogen production;water electrolysis1012|0|0更新时间:2025-10-22
摘要:The production of green hydrogen using renewable energy to synthesize green methanol can solve the problem of renewable energy consumption and is also one of the important ways to reduce carbon dioxide emissions. In order to design a green methanol project and assess its technical and economic feasibility, a case study of a green methanol project in Northeast China was conducted. The technical route and process flow of biomass gasification coupled with renewable energy hydrogen production for green methanol synthesis were evaluated. Considering the volatility of renewable energy generation, time-period scheduling control was applied. With the goal of optimizing the project’s economic performance, the real-time power balance method was simulated and matched, resulting in a hydrogen production capacity of 52000 m3/h for a 500 MW wind power installation. Additionally, the effective hydrogen storage capacity is 2210000 m3, and the green methanol production capacity is 160000 t/a. Under the given boundary conditions, internal rate of return (IRR), payback period, total return on investment,and net profit margin on equity were selected as the economic indicators. With a project financial IRR (after tax) of 6%, the green methanol price is calculated to be 5324.06 CNY/t. Additionally, the payback period (after tax) is 12.25 a, the financial IRR on equity is 11.91%, the total return on investment is 4.19%, and the net profit margin on equity is 12.59%. The project is economically viable. The proposed project plan is feasible, meets relevant standards and engineering requirements, and can provide a reference for future green methanol project design.关键词:renewable energy;green methanol;biomass gasification;hydrogen production;water electrolysis1012|0|0更新时间:2025-10-22 -
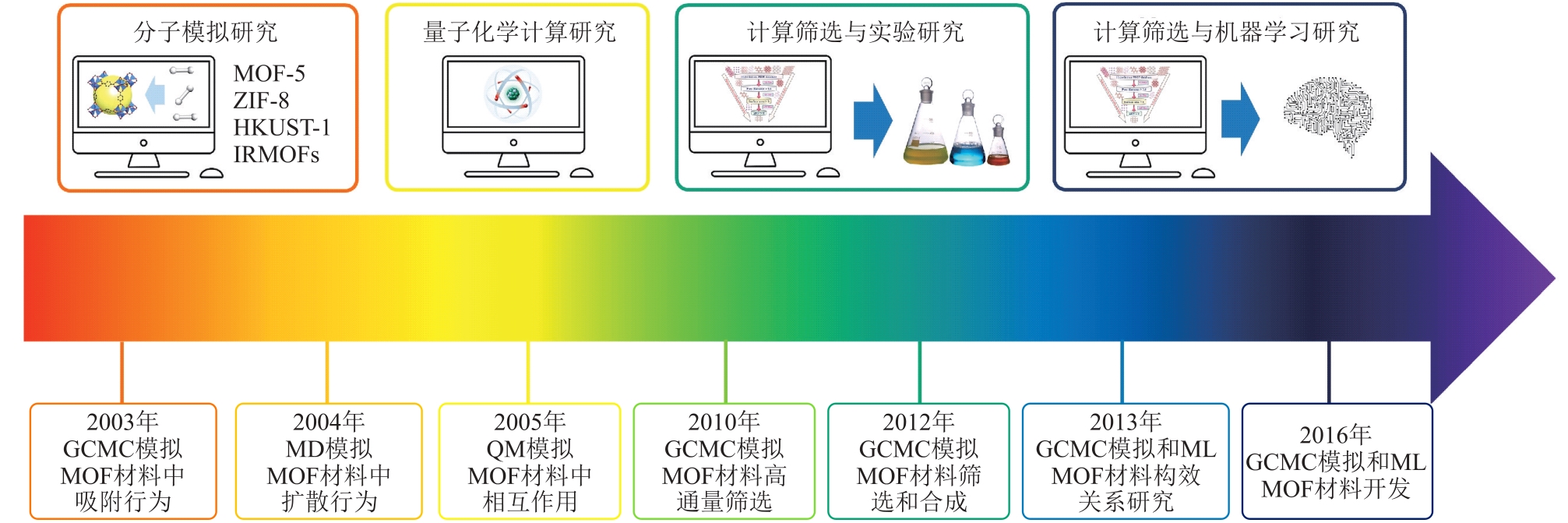 摘要:As a kind of nanoporous crystalline material, metal-organic framework (MOF) are constructed by self-assembly of metal nodes and organic ligands. Due to the advantages such as large specific surface area and high structural tunability, MOF has shown great application potential in light hydrocarbon separation fields, including heavy hydrocarbon removal in natural gas, alkane and alkene separation and acetylene purification. However, realizing efficient “on-demand” design of target application materials from tens of thousands of MOF remains a huge challenge. The computer-aided technology provides an efficient tool to easily realize separation performance prediction of a large number of MOF through theoretical calculations and data-driven approaches. Based on cutting-edge research achievements in computer-aided MOF design, the application advances in multiscale computing methods (quantum chemistry calculation, molecular dynamics simulation and Monte Carlo simulation) in the study of MOF for light hydrocarbon adsorption/separation were systematically reviewed, and case studies of emerging machine learning methods to assist MOF development were discussed. Finally, by combining the technical characteristics of multiscale computing and data-driven methods, the future development directions of computer-aided MOF design were prospected.关键词:metal-organic framework;light hydrocarbon separation;multiscale computing;machine learning40|0|0更新时间:2025-10-22
摘要:As a kind of nanoporous crystalline material, metal-organic framework (MOF) are constructed by self-assembly of metal nodes and organic ligands. Due to the advantages such as large specific surface area and high structural tunability, MOF has shown great application potential in light hydrocarbon separation fields, including heavy hydrocarbon removal in natural gas, alkane and alkene separation and acetylene purification. However, realizing efficient “on-demand” design of target application materials from tens of thousands of MOF remains a huge challenge. The computer-aided technology provides an efficient tool to easily realize separation performance prediction of a large number of MOF through theoretical calculations and data-driven approaches. Based on cutting-edge research achievements in computer-aided MOF design, the application advances in multiscale computing methods (quantum chemistry calculation, molecular dynamics simulation and Monte Carlo simulation) in the study of MOF for light hydrocarbon adsorption/separation were systematically reviewed, and case studies of emerging machine learning methods to assist MOF development were discussed. Finally, by combining the technical characteristics of multiscale computing and data-driven methods, the future development directions of computer-aided MOF design were prospected.关键词:metal-organic framework;light hydrocarbon separation;multiscale computing;machine learning40|0|0更新时间:2025-10-22 -
 摘要:Traditional acid gas purification technologies face issues such as high energy consumption and failure to meet the purification standards for carbonyl sulfide (COS) and hydrogen sulfide (H2S). Using Aspen process flow simulation software, a purification unit (alkanolamine process) that incorporates a carbonyl sulfide catalytic hydrolysis device was optimized. The effects of operating parameters such as solution circulation rate, solution inlet temperature, absorption pressure and packing height on gas purification efficiency and energy consumption were investigated. The results show that appropriately reducing the solution circulation rate, solution inlet temperature, absorption pressure and packing height can decrease CO2 absorption, thereby improving the yield of the purified gas. Under optimized conditions (solution circulation rate of 262 m3/h, solution inlet temperature of 40 ℃, absorption pressure of 4.5 MPa and packing height of 15.5 m), complete removal of H2S from acid gas was achieved. The CO2 content (mole fraction) in the purified gas can be reduced to 2.95%, and the regeneration energy consumption can be controlled at 3.4 GJ/t. The annual savings in low-pressure steam amounted to 28.8 × 104 t, leading to a significant reduction in energy consumption.关键词:gas purification;process simulation;amine solutions;optimization of operating parameters126|0|0更新时间:2025-10-22
摘要:Traditional acid gas purification technologies face issues such as high energy consumption and failure to meet the purification standards for carbonyl sulfide (COS) and hydrogen sulfide (H2S). Using Aspen process flow simulation software, a purification unit (alkanolamine process) that incorporates a carbonyl sulfide catalytic hydrolysis device was optimized. The effects of operating parameters such as solution circulation rate, solution inlet temperature, absorption pressure and packing height on gas purification efficiency and energy consumption were investigated. The results show that appropriately reducing the solution circulation rate, solution inlet temperature, absorption pressure and packing height can decrease CO2 absorption, thereby improving the yield of the purified gas. Under optimized conditions (solution circulation rate of 262 m3/h, solution inlet temperature of 40 ℃, absorption pressure of 4.5 MPa and packing height of 15.5 m), complete removal of H2S from acid gas was achieved. The CO2 content (mole fraction) in the purified gas can be reduced to 2.95%, and the regeneration energy consumption can be controlled at 3.4 GJ/t. The annual savings in low-pressure steam amounted to 28.8 × 104 t, leading to a significant reduction in energy consumption.关键词:gas purification;process simulation;amine solutions;optimization of operating parameters126|0|0更新时间:2025-10-22 -
Study on low-temperature catalytic oxidation of toluene by Cu-doped MnO
x : Synergetic effect of Cu-Mn 摘要:The environmental pollution caused by emission of volatile organic compounds (VOCs) has become increasingly severe, and the development of catalysts with low-temperature catalytic activity for VOCs treatment has become a research focus in the field of catalytic purification. To address the insufficient low-temperature activity of MnOx catalysts in toluene oxidation, the Cu-doping strategy was employed to regulate the structural and surface properties of MnOx and construct Mn-Ov-Cu (Ov represents oxygen vacancies) synergistic active sites. A series of Cu-doped MnOx catalysts were synthesized via hydrothermal method, and their phase compositions, functional groups, surface compositions and chemical states were characterized using XRD, FT-IR, XPS and so on. The catalytic oxidation performances of the catalysts were studied using MnOx and CuOx as control groups. The results show that Cu-doping can promote the formation of Cu-Mn solid solutions, reduce the crystallinities of catalysts, increase the specific surface areas and the numbers of structural defect, thereby facilitating the exposure of active sites and the adsorption of reactants. Among the catalysts, for the catalyst with n(Cu):n(Mn) of 1:9 (1Cu9MnOx), the proportion (molar fraction) of surface adsorbed oxygen in surface oxygen species is 32.5%. Under the conditions of toluene (mole fraction of 5 × 10-4) and air (O2 volume fraction of 20%) as main components in reaction gas, the total flow rate of reaction gas of 100 mL/min and space velocity of 60000 h-1, the toluene conversion rate of 1Cu9MnOx at 230 ℃ is 90%, which is superior to that of MnOx and CuOx.关键词:toluene;catalytic oxidation;Cu-doped MnOx;oxygen vacancies;synergetic effect17|0|0更新时间:2025-10-22
摘要:The environmental pollution caused by emission of volatile organic compounds (VOCs) has become increasingly severe, and the development of catalysts with low-temperature catalytic activity for VOCs treatment has become a research focus in the field of catalytic purification. To address the insufficient low-temperature activity of MnOx catalysts in toluene oxidation, the Cu-doping strategy was employed to regulate the structural and surface properties of MnOx and construct Mn-Ov-Cu (Ov represents oxygen vacancies) synergistic active sites. A series of Cu-doped MnOx catalysts were synthesized via hydrothermal method, and their phase compositions, functional groups, surface compositions and chemical states were characterized using XRD, FT-IR, XPS and so on. The catalytic oxidation performances of the catalysts were studied using MnOx and CuOx as control groups. The results show that Cu-doping can promote the formation of Cu-Mn solid solutions, reduce the crystallinities of catalysts, increase the specific surface areas and the numbers of structural defect, thereby facilitating the exposure of active sites and the adsorption of reactants. Among the catalysts, for the catalyst with n(Cu):n(Mn) of 1:9 (1Cu9MnOx), the proportion (molar fraction) of surface adsorbed oxygen in surface oxygen species is 32.5%. Under the conditions of toluene (mole fraction of 5 × 10-4) and air (O2 volume fraction of 20%) as main components in reaction gas, the total flow rate of reaction gas of 100 mL/min and space velocity of 60000 h-1, the toluene conversion rate of 1Cu9MnOx at 230 ℃ is 90%, which is superior to that of MnOx and CuOx.关键词:toluene;catalytic oxidation;Cu-doped MnOx;oxygen vacancies;synergetic effect17|0|0更新时间:2025-10-22 -
 摘要:In the CO2 direct air capture (DAC) technology, finding low-cost and humidity-stable moisture-swing adsorption materials is crucial for enhancing the practicality and environmental adaptability of the adsorbents. The porous composite humidity adsorbent K2CO3/AC was prepared by loading potassium carbonate on activated carbon (AC). The physical properties and CO2 adsorption performance of the adsorbents based on wood, coal, and coconut shell activated carbon were studied and compared. Furthermore, hydrophilic and hydrophobic modifications on the low-cost wood activated carbon based adsorbent were conducted and the impact of ambient humidity on adsorbent performance was investigated. The results show that the coconut shell activated carbon based composite moisture adsorbent has the best humidity stability, and its CO2 adsorption capacity reaches the maximum value (0.85 mmol/g) at room temperature and relative humidity of 30%. This is primarily attributed to its superior pore structure and relatively high hydrophobicity. Besides, the hydrophilic modified wood activated carbon-based adsorbent exhibits a rapid decline in adsorption performance as humidity increases, whereas the hydrophobic modified adsorbent demonstrates better humidity stability, with optimal CO2 adsorption performance reaching 0.81 mmol/g at room temperature and relative humidity of 30%. Enhancing the CO2 adsorption performance and environmental adaptability of moisture-swing adsorbents through hydrophobic modification holds great potential for promoting the wider application of DAC technology.关键词:CO2;moisture-swing adsorption;activated carbon;direct air capture263|0|0更新时间:2025-10-22
摘要:In the CO2 direct air capture (DAC) technology, finding low-cost and humidity-stable moisture-swing adsorption materials is crucial for enhancing the practicality and environmental adaptability of the adsorbents. The porous composite humidity adsorbent K2CO3/AC was prepared by loading potassium carbonate on activated carbon (AC). The physical properties and CO2 adsorption performance of the adsorbents based on wood, coal, and coconut shell activated carbon were studied and compared. Furthermore, hydrophilic and hydrophobic modifications on the low-cost wood activated carbon based adsorbent were conducted and the impact of ambient humidity on adsorbent performance was investigated. The results show that the coconut shell activated carbon based composite moisture adsorbent has the best humidity stability, and its CO2 adsorption capacity reaches the maximum value (0.85 mmol/g) at room temperature and relative humidity of 30%. This is primarily attributed to its superior pore structure and relatively high hydrophobicity. Besides, the hydrophilic modified wood activated carbon-based adsorbent exhibits a rapid decline in adsorption performance as humidity increases, whereas the hydrophobic modified adsorbent demonstrates better humidity stability, with optimal CO2 adsorption performance reaching 0.81 mmol/g at room temperature and relative humidity of 30%. Enhancing the CO2 adsorption performance and environmental adaptability of moisture-swing adsorbents through hydrophobic modification holds great potential for promoting the wider application of DAC technology.关键词:CO2;moisture-swing adsorption;activated carbon;direct air capture263|0|0更新时间:2025-10-22 -
 摘要:Amino acid salt solutions, as carbon dioxide absorbents, have garnered significant attention in the field of CO2 capture due to their advantages of rapid absorption rate, high absorption capacity, and low toxicity. Using the mixed amino acid salt solution with mass fraction of 15% N-methyldiethanolamine (MDEA), 15% monoethanolamine, and 5% potassium lysine, respectively, as the CO2 absorbent, a neural network time series method based on backpropagation (BP) algorithm was utilized to predict CO2 absorption data under varying temperatures during 400 min, and the predicted data was systematically compared with experimental data. The results show that the optimal hyperparameters for the prediction model are as follows: 4-7-1 architecture for input-hidden-output layers, initial damping factor of 0.05, Tansig function as activation function, and the algorithm of Particle Swarm Optimization (PSO)-BP neural network algorithm integrated with Levenberg-Marquardt iterative optimization. After multiple data simulations, the model achieves an average mean squared error of 1.8289 × 10-11. With the optimal hyperparameters, using the model with the verification group proportion of 70% in verification group and training group and the determination coefficient of 0.9836, the maximum relative error between predicted and experimental absorption capacity values reaches 2.031% at 40 °C during 400 min.关键词:carbon dioxide absorption;amino acid salt solution;neural network;time series method;data prediction128|0|0更新时间:2025-10-22
摘要:Amino acid salt solutions, as carbon dioxide absorbents, have garnered significant attention in the field of CO2 capture due to their advantages of rapid absorption rate, high absorption capacity, and low toxicity. Using the mixed amino acid salt solution with mass fraction of 15% N-methyldiethanolamine (MDEA), 15% monoethanolamine, and 5% potassium lysine, respectively, as the CO2 absorbent, a neural network time series method based on backpropagation (BP) algorithm was utilized to predict CO2 absorption data under varying temperatures during 400 min, and the predicted data was systematically compared with experimental data. The results show that the optimal hyperparameters for the prediction model are as follows: 4-7-1 architecture for input-hidden-output layers, initial damping factor of 0.05, Tansig function as activation function, and the algorithm of Particle Swarm Optimization (PSO)-BP neural network algorithm integrated with Levenberg-Marquardt iterative optimization. After multiple data simulations, the model achieves an average mean squared error of 1.8289 × 10-11. With the optimal hyperparameters, using the model with the verification group proportion of 70% in verification group and training group and the determination coefficient of 0.9836, the maximum relative error between predicted and experimental absorption capacity values reaches 2.031% at 40 °C during 400 min.关键词:carbon dioxide absorption;amino acid salt solution;neural network;time series method;data prediction128|0|0更新时间:2025-10-22 -
 摘要:The non-catalytic partial oxidation of natural gas has been widely industrialized, but further cost reduction remains a key challenge for the technology. Based on computational fluid dynamics and the GRI-Mech 3.0 chemical reaction mechanism, numerical simulations of acetylene production via partial oxidation of natural gas with air were conducted, and its feasibility in terms of chemical reactions and process design was investigated. The results indicate that nitrogen does not participate in the reaction. Under conditions of the preheating temperature of 923 K, oxygen-to-carbon ratio (n(O2)/n(CH4)) of 0.70 and feed velocity of 200 m/s to 300 m/s, the mole fraction of acetylene at the reactor outlet can reach 8.9%, which is higher than the 7.8% observed in industrial production. The hydrogen-to-carbon monoxide ratio (n(H2)/n(CO)) of the generated syngas is 1.8, slightly lower than that obtained using pure oxygen as the oxidant. Although the product separation process is more complex when air is used as the oxidant, it still shows good application prospects due to the elimination of air separation units and associated high-energy-consuming operations.关键词:natural gas;partial oxidation;air;acetylene;computational fluid dynamics41|0|0更新时间:2025-10-22
摘要:The non-catalytic partial oxidation of natural gas has been widely industrialized, but further cost reduction remains a key challenge for the technology. Based on computational fluid dynamics and the GRI-Mech 3.0 chemical reaction mechanism, numerical simulations of acetylene production via partial oxidation of natural gas with air were conducted, and its feasibility in terms of chemical reactions and process design was investigated. The results indicate that nitrogen does not participate in the reaction. Under conditions of the preheating temperature of 923 K, oxygen-to-carbon ratio (n(O2)/n(CH4)) of 0.70 and feed velocity of 200 m/s to 300 m/s, the mole fraction of acetylene at the reactor outlet can reach 8.9%, which is higher than the 7.8% observed in industrial production. The hydrogen-to-carbon monoxide ratio (n(H2)/n(CO)) of the generated syngas is 1.8, slightly lower than that obtained using pure oxygen as the oxidant. Although the product separation process is more complex when air is used as the oxidant, it still shows good application prospects due to the elimination of air separation units and associated high-energy-consuming operations.关键词:natural gas;partial oxidation;air;acetylene;computational fluid dynamics41|0|0更新时间:2025-10-22 -
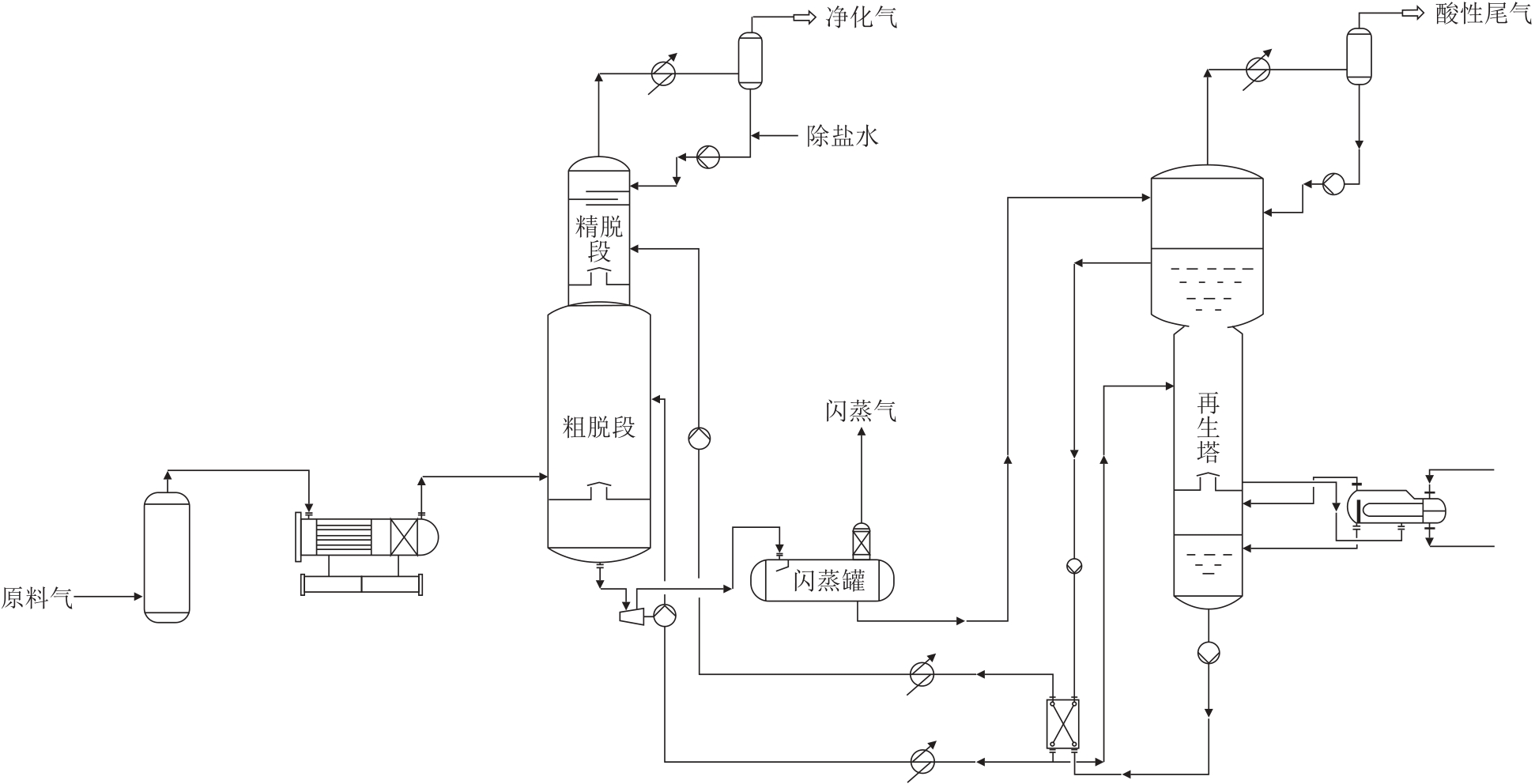 摘要:For the decarbonization of high CO2 content natural gas, the conventional semi-lean liquid process suffers from insufficient decarbonization depth and excessively high regeneration energy consumption. To improve decarbonization accuracy and reduce regeneration energy consumption, a novel dual-tower absorption semi-lean liquid decarbonization process was proposed. A process model of the new scheme was established using Aspen HYSYS software, and a single-factor sensitivity analysis was conducted to investigate the effects of key parameters, including amine liquid circulation rate, semi-lean liquid split ratio and regeneration temperature, on decarbonization performance and regeneration energy consumption, followed by parameter optimization. Under the optimal process conditions (raw gas with CO2 volume fraction of 23.5%, amine liquid circulation rate of 1195 m3/h, semi-lean liquid split ratio of 75% and regeneration temperature of 120 ℃), the new process was compared with the conventional process. The results show that, compared with the conventional process, the new process increases the purified gas flow by 21.63 × 104 m3/d, corresponding to an economic benefit of 59.05 × 104 CNY/d. Moreover, the sizes of the absorption tower, regeneration tower and rich liquid flash tank are all significantly reduced. The regeneration and cooling energy consumption of the new process are reduced by 4.836% and 4.572%, respectively, demonstrating a notable energy-saving effect.关键词:decarbonization;dual-tower absorption;semi-lean liquid;amine liquid circulation rate;semi-lean liquid split ratio;regeneration energy consumption2|0|0更新时间:2025-10-22
摘要:For the decarbonization of high CO2 content natural gas, the conventional semi-lean liquid process suffers from insufficient decarbonization depth and excessively high regeneration energy consumption. To improve decarbonization accuracy and reduce regeneration energy consumption, a novel dual-tower absorption semi-lean liquid decarbonization process was proposed. A process model of the new scheme was established using Aspen HYSYS software, and a single-factor sensitivity analysis was conducted to investigate the effects of key parameters, including amine liquid circulation rate, semi-lean liquid split ratio and regeneration temperature, on decarbonization performance and regeneration energy consumption, followed by parameter optimization. Under the optimal process conditions (raw gas with CO2 volume fraction of 23.5%, amine liquid circulation rate of 1195 m3/h, semi-lean liquid split ratio of 75% and regeneration temperature of 120 ℃), the new process was compared with the conventional process. The results show that, compared with the conventional process, the new process increases the purified gas flow by 21.63 × 104 m3/d, corresponding to an economic benefit of 59.05 × 104 CNY/d. Moreover, the sizes of the absorption tower, regeneration tower and rich liquid flash tank are all significantly reduced. The regeneration and cooling energy consumption of the new process are reduced by 4.836% and 4.572%, respectively, demonstrating a notable energy-saving effect.关键词:decarbonization;dual-tower absorption;semi-lean liquid;amine liquid circulation rate;semi-lean liquid split ratio;regeneration energy consumption2|0|0更新时间:2025-10-22
0

